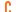- Previous close
0.0550 - Open
0.0560 - Bid 0.0500 x --
- Ask 0.0550 x --
- Day's range
0.0560 - 0.0560 - 52-week range
0.0500 - 0.0940 - Volume
100 - Avg. Volume
468,496 - Market cap (intra-day)
57.39M - Beta (5Y monthly) 0.78
- PE ratio (TTM)
-- - EPS (TTM)
-0.0600 - Earnings date 7 Aug 2024
- Forward dividend & yield --
- Ex-dividend date --
- 1y target est
--
The Trendlines Group Ltd. is an innovation commercialization company and a private equity and venture capital firm specializing in incubation, seed, start-up, early venture and late venture investments. The firm seeks to invest in food, medtech, clean technology, agrifood, agritechnology, lifesciences with a focus on medical devices, biotechnology, and pharmaceuticals, security, medical, and information technology companies. It considers investments in Israel and Singapore. The firm offers business incubation, partner and channel development, market strategies, business plans, research and development and commercialization activities, business development, finance and funding strategies, administrative services and marketing communications services to entrepreneurs. The firm prefers to exit its investments through merger and acquisition transactions, listing on public stock exchanges, or other dispositions of holdings. It leverages its own capital with government funding to mitigate risk in creating and developing new businesses. It is involved in all aspects of its portfolio companies from technology development to business building. The Trendlines Group Ltd. was founded in 1993 and is based in M.P. Misgav, Israel with additional offices in Tel Aviv, Israel; Beijing, China; and Singapore, Singapore.
www.trendlines.com24
Full-time employees
31 December
Fiscal year ends
Financial Services
Sector
Asset Management
Industry
Recent news: 42T.SI
View morePerformance overview: 42T.SI
Trailing total returns as of 25/10/2024, which may include dividends or other distributions. Benchmark is
.YTD return
1-year return
3-year return
5-year return
Compare to: 42T.SI
Select to analyse similar companies using key performance metrics; select up to four stocks.
Statistics: 42T.SI
View moreValuation measures
Market cap
56.37M
Enterprise value
53.59M
Trailing P/E
--
Forward P/E
--
PEG ratio (5-yr expected)
--
Price/sales (ttm)
12.59
Price/book (mrq)
0.56
Enterprise value/revenue
12.55
Enterprise value/EBITDA
--
Financial highlights
Profitability and income statement
Profit margin
0.00%
Return on assets (ttm)
-23.26%
Return on equity (ttm)
-41.88%
Revenue (ttm)
-27M
Net income avi to common (ttm)
-36.91M
Diluted EPS (ttm)
-0.0600
Balance sheet and cash flow
Total cash (mrq)
6.38M
Total debt/equity (mrq)
5.59%
Levered free cash flow (ttm)
-21.62M