Bristol Myers' (BMY) Krazati Gets FDA Nod for Colorectal Cancer
Bristol Myers Squibb BMY announced that the FDA has approved a label expansion of its oncology drug Krazati (adagrasib).
The FDA granted accelerated approval to Krazati, in combination with Erbitux (cetuximab), as a targeted treatment option for adult patients with KRASG12C-mutated locally advanced or metastatic colorectal cancer (CRC).
The FDA’s approval for this indication was on an accelerated basis, based on objective response rate (ORR) and duration of response (DOR) results. Continued approval for this indication may be contingent upon verification and description of a clinical benefit in a confirmatory trial.
The approval is based on positive results from cohorts of the open-label phase I/II KRYSTAL-1 study, wherein Krazati, in combination with Erbitux, showed an ORR of 34% in pretreated patients with locally advanced or metastatic CRC harboring a KRASG12C mutation. The median DOR, one of the secondary endpoints, was 5.8 months.
The FDA had granted breakthrough therapy designation to Krazati, in combination with Erbitux, for patients with KRASG12C-mutated advanced CRC whose cancer has progressed following prior treatment with certain chemotherapy and an anti-VEGF therapy.
Per BMY, CRC with a KRASG12C mutation occurs in approximately 3-4% of CRC patients and is challenging to treat. The latest FDA approval of Krazati, in combination with Erbitux, will provide a new treatment option to these patients when their tumors do not respond well to the prior therapies.
The drug was added to BMY’s portfolio following its acquisition of Mirati Therapeutics in January 2024.
Krazati has also obtained accelerated approval for the treatment of adult patients with KRASG12C-mutated locally advanced or metastatic non-small cell lung cancer (NSCLC), as determined by an FDA-approved test, who have received at least one prior systemic therapy. The accelerated approval was based on ORR and DOR.
The drug is being evaluated as monotherapy and in combination with other anti-cancer therapies in patients with advanced KRASG12C-mutated solid tumors, including NSCLC and colorectal cancer.
Shares of Bristol Myers have lost 18.3% year to date compared with the industry's decline of 5.9%.
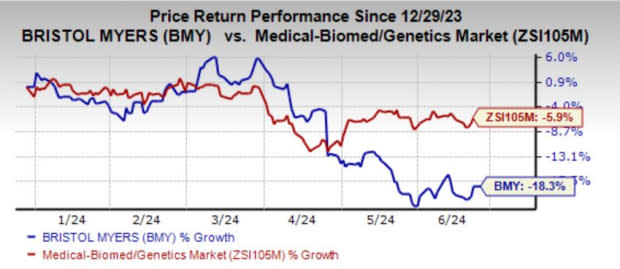
Image Source: Zacks Investment Research
BMY is looking to expand its new product portfolio amid generic challenges for Revlimid and Eliquis. Approval of new drugs and label expansion of the existing ones are important for BMY and the going has been good in that regard.
The FDA recently approved a label expansion of BMY’s another lung cancer drug Augtyro (repotrectinib).
The regulatory body granted accelerated approval to Augtyro for the treatment of adult and pediatric patients aged 12 years and above with solid tumors that have a neurotrophic tyrosine receptor kinase gene fusion, are locally advanced or metastatic or where surgical resection is likely to result in severe morbidity, and have progressed following treatment or have no satisfactory alternative therapy.
BMY had earlier obtained regulatory approvals for the label expansion of its two CAR T cell therapies — Abecma and Breyanzi. The company also received the European Commission's approval for the label expansion of Reblozyl (luspatercept).
Zacks Rank and Stocks to Consider
BMY currently has a Zacks Rank #3 (Hold).
Some better-ranked stocks in the biotech sector are Ligand Pharmaceuticals LGND, ALX Oncology Holdings ALXO and Minerva Neurosciences, Inc. NERV, each carrying a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
In the past 30 days, the Zacks Consensus Estimate for Ligand’s 2024 earnings per share (EPS) has increased 16 cents to $4.71. During the same time frame, the consensus estimate for 2025 EPS has increased 70 cents to $5.90. Year to date, shares of LGND have risen 12.2%.
Ligand beat on earnings in each of the trailing four quarters, delivering an average surprise of 56.02%.
In the past 60 days, the Zacks Consensus Estimate for ALX Oncology’s 2024 loss per share has narrowed from $3.33 to $2.89. During the same period, the consensus estimate for 2025 loss per share has narrowed from $2.85 to $2.73.
ALX Oncology beat on earnings in two of the trailing four quarters and missed the mark in the other two, delivering an average negative surprise of 8.83%.
In the past 60 days, estimates for Minerva Neurosciences’ 2024 loss per share have narrowed from $3.57 to $1.89. Loss per share estimate for 2025 has narrowed from $4.54 to $3.60 during the same time frame.
NERV’s earnings beat estimates in one of the trailing four quarters and missed the same in the other three, the average negative surprise being 54.43%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Bristol Myers Squibb Company (BMY) : Free Stock Analysis Report
Ligand Pharmaceuticals Incorporated (LGND): Free Stock Analysis Report
Minerva Neurosciences, Inc (NERV) : Free Stock Analysis Report
ALX Oncology Holdings Inc. (ALXO) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 