Biotech Stock Roundup: ITCI Up on Study Data, ZNTL, OVID Down on Updates & More News
It was a busy week for the biotech sector with quite a few important pipeline and regulatory updates. Among these, Intra-Cellular Therapies ITCI soared on study data, while Zentalis Pharmaceuticals, Inc. ZNTL tanked on a regulatory setback.
Recap of the Week’s Most Important Stories:
ITCI Surges on Study Success: Intra-Cellular Therapies reported positive top-line results from Study 502, the second late-stage study that evaluated lumateperone 42 mg for the treatment of major depressive disorder (MDD). The phase III study evaluating lumateperone 42 mg, given once daily as an adjunctive therapy to antidepressants for MDD, met the primary endpoint. A statistically significant and clinically meaningful reduction in the Montgomery-Åsberg Depression Rating Scale (MADRS) total score was observed compared with placebo at week six.
Intra-Cellular also reported that the key secondary endpoint was also achieved by demonstrating a statistically significant and clinically meaningful reduction in the Clinical Global Impression Scale score, which is a measure of the severity of the illness compared with placebo at week six.
Additionally, feedback from MDD patients, as measured by the Quick Inventory of Depressive Symptomatology Self Report (QIDS-SR-16), demonstrated that lumateperone 42 mg robustly improved depressive symptoms. Per the company, the success of both the phase III studies, Study 501 and Study 502, forms the basis for a regulatory filing seeking the label expansion of lumateperone as an adjunctive treatment of MDD. Intra-Cellular expects to submit a supplemental new drug application for lumateperone in the United States (to treat the MDD indication) in the second half of 2024.
Zentalis Plunges on Setback: Zentalis Pharmaceuticals announced that the FDA has placed a partial clinical hold on three studies of its lead pipeline candidate, azenosertib. Shares of this clinical-stage biopharmaceutical company plunged on the news.
The phase I ZN-c3-001 dose-escalation study was investigating azenosertib for treating solid tumors, while the phase II ZN-c3-005 DENALI study was evaluating azenosertib in platinum-resistant ovarian cancer (PROC). The phase II ZN-c3-004 TETON study was evaluating azenosertib for the treatment of uterine serous carcinoma.
The FDA placed a partial clinical hold on all these studies after two patients in the DENALI study died due to presumed sepsis. Zentalis remains focused on resolving this clinical hold at the earliest. ZNTL has already completed enrollment in cohort 1b of the DENALI study. Overall efficacy and safety data from the same is expected to be announced later in 2024. Zentalis also plans to present data from the ZN-c3-001 and the MAMMOTH studies later this year.
OVID Plummets on Pipeline Update: Shares of Ovid Therapeutics OVID crashed after it announced that the treatment with soticlestat, its Takeda-partnered investigational epilepsy drug, failed to meet the primary endpoint of two pivotal late-stage studies. Ovid’s partner Takeda evaluated soticlestat for treating two rare and severe forms of epilepsy, namely Dravet syndrome (DS) and Lennox-Gastaut syndrome (LGS), in the late-stage SKYLINE and SKYWAY studies, respectively.
While the SKYLINE study narrowly missed out on its primary endpoint of reducing convulsive seizure frequency in DS patients, the SKYWAY study did not meet its primary endpoint of reducing major motor drop seizures in LGS patients. Some pre-specified subgroups of patients in both studies showed significant treatment effects on the primary and secondary efficacy endpoints.
OVID sold its rights in soticlestat to Takeda in 2021 and received an upfront payment of $196 million. It was also eligible to receive milestone payments of up to $660 million and tiered royalties on potential net product sales of up to 20%. These payments would have likely benefited Ovid, which currently has no marketed product of its own. The stock plummeted, possibly due to the potential loss of these milestone payments.
MRNA’s Vaccine Data: Moderna MRNA announced positive results from the phase III study evaluating mRNA-1283, its next-generation refrigerator-stable COVID-19 vaccine, in individuals aged 12 years and older. The study achieved its primary efficacy endpoint, demonstrating the non-inferior vaccine efficacy of mRNA-1283 against COVID-19 when compared to mRNA-1273.222, Moderna’s previously approved bivalent Omicron BA.4/BA.5-targeting COVID-19 vaccine. A higher efficacy was also observed in these study participants when compared to mRNA-1273.222.
Moderna had previously reported positive interim immunogenicity results from the study in March. Individuals who received mRNA-1283 generated a higher immune response against both the Omicron BA.4/BA.5 and original strains of SARS-CoV-2 when compared to mRNA-1273.222.
Update From Amgen: Amgen AMGN announced that the FDA has granted approval to its leukemia drug, Blincyto (blinatumomab), for the treatment of adult and pediatric patients aged one month or older with CD19-positive Philadelphia chromosome-negative B-cell precursor acute lymphoblastic leukemia (B-ALL) in the consolidation phase, regardless of measurable residual disease (MRD) status.
The approval for consolidation treatment was based on data from the phase III E1910 study. Data from the study showed that Blincyto, added to multiphase consolidation chemotherapy, reduced the risk of death by 58%, showing superior overall survival versus chemotherapy alone.
Blincyto is a key contributor to Amgen’s top line. The approval for consolidation treatment marks the third indication for Blincyto. It is already approved for treating CD19-positive B-ALL in adult and pediatric patients aged one month and older with MRD greater than or equal to 0.1% in the first or second complete remission and those with relapsed or refractory disease.
AMGN currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Performance
The Nasdaq Biotechnology Index has lost 2.58% in the past four trading sessions and Moderna’s shares have fallen 9.78% during the same time frame. In the past six months, shares of MRNA have rallied 49.59%. (See the last biotech stock roundup here: Biotech Stock Roundup: GSK’s Litigation Update, BIIB’s Drug Approval & Other Updates)
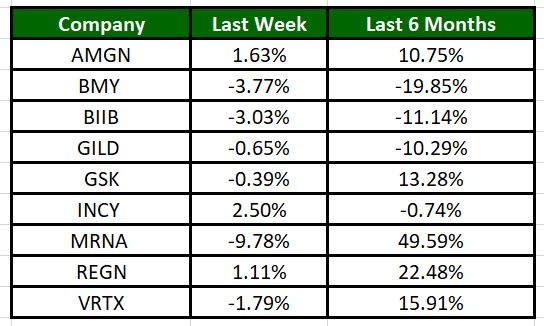
Image Source: Zacks Investment Research
What's Next in Biotech?
Stay tuned for more pipeline updates.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Amgen Inc. (AMGN) : Free Stock Analysis Report
Moderna, Inc. (MRNA) : Free Stock Analysis Report
Intra-Cellular Therapies Inc. (ITCI) : Free Stock Analysis Report
Ovid Therapeutics (OVID) : Free Stock Analysis Report
Zentalis Pharmaceuticals, Inc. (ZNTL) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 