Ultragenyx (RARE) Q1 Earnings and Revenues Miss Estimates
Ultragenyx Pharmaceutical Inc. RARE incurred a loss of $2.33 per share in first-quarter 2023, wider than the Zacks Consensus Estimate of a loss of $1.97. The company reported a $2.19 per share loss in the year-ago quarter.
Ultragenyx’s total revenues amounted to $100 million in the first quarter, up 25.7% year over year. The top line, however, missed the Zacks Consensus Estimate of $105 million.
Shares of Ultragenyx have lost 1.2% in the year so far compared with the industry’sdecline of 6.2%.
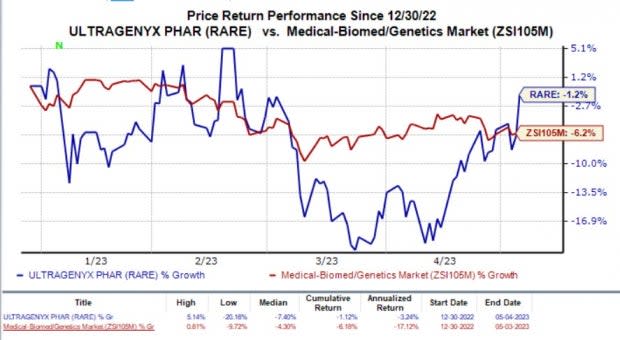
Image Source: Zacks Investment Research
Ultragenyx markets four drugs, namely Crysvita, Mepsevii, Dojolvi and Evkeeza. Crysvita is approved for treating X-linked hypophosphatemia, an inherited disorder and tumor-induced osteomalacia, an ultra-rare disease. Mepsevii is approved to treat Mucopolysaccharidosis VII (MPS VII), also known as Sly syndrome. Dojolvi was approved in June 2020 for all forms of long-chain fatty acid oxidation disorders (LC-FAOD).
In January 2022, Ultragenyx announced a license and collaboration agreement with Regeneron Pharmaceuticals REGN. Per the deal, Ultragenyx has obtained the rights to develop, commercialize and distribute Evkeeza (evinacumab) outside the United States. The regions include the European Economic Area. The collaboration with Regeneron for Evkeeza gives Ultragenyx a fourth-approved product that adds to the top line. However, Regeneron will continue to solely commercialize Evkeeza in the United States.
Quarter in Detail
Crysvita’s total revenues were $76 million, up 28% year over year, driven by increased demand for approved indications. Crysvita revenues from North America were $49.9 million in the first quarter, up 10.4% from the year-ago quarter. Total product revenues related to Crysvita from Latin America amounted to $21.2 million, up 126% from the year-ago quarter’s figure. Total royalty revenues related to the sales of Crysvita in the European region were $4.9 million. Ultragenyx sold its Crysvita rights in the European territory to Royalty Pharma in December 2019.
Mepsevii product revenues were $8.5 million in the first quarter compared with $4.9 million reported in the year-ago quarter. Dojolvi product revenues were $14.3 million compared with $12.4 million in the year-ago quarter, driven by strong new patient demand. Evkeeza recorded sales of $0.2 million in the reported quarter.
Collaboration revenues of $1.5 million, from technology transfer as a part of a collaboration and license agreement with Daiichi Sankyo were also included in total revenues in the reported quarter. collaboration and license revenues in the year-ago quarter were $3.2 million.
Operating expenses increased 17.6% to $254.6 million in the first quarter. Operating expenses for the reported quarter include research and development expenses of $165.7 million (up 15.7%) and selling, general and administrative expenses of $76.6 million (up 13.8%).
Cash, cash equivalents and marketable debt securities amounted to $714.6 million as of quarter-ended Mar 31, 2023, compared with $896.7 million as of Dec 31, 2022.
2023 Guidance Reaffirmed
Ultragenyx expects total revenues in 2023 between $425 million and $450 million. Crysvita revenues are expected in the range of $325-$340 million (including all regions where Ultragenyx will recognize revenues, including the royalties in Europe, which have been ongoing and the royalties in North America, which began in April 2023). Dojolvi revenues are expected between $65 million and $75 million.
Operating expenses are expected to decrease in 2023 (net cash used in operations is expected to be less than $400 million) as the company manages headcount and increases operational leverage while executing high-value programs.
Pipeline Updates
Ultragenyx is evaluating GTX-102 for treating Angelman syndrome, a rare neurogenetic disorder, in a phase I/II study that continues dose exploration in the United States.
Currently, Ultragenyx is dosing patients in a phase II/III Orbit study of UX143 for the treatment of osteogenesis imperfecta (OI) in patients aged between five and less than 26 years. Enrollment in the phase 2 single ascending dose portion of the study is complete and data is expected in mid-2023. Phase 3 portion of the study is expected to be initiated in mid-2023. The company has decided to not enroll patients in the multiple ascending dose cohorts currently, to focus greater resources on its later-stage programs.
Ultragenyx is also planning to initiate another study on UX143 in pediatric patients (> 5 years) with OI, in the second quarter of 2023.
Ultragenyx Pharmaceutical Inc. Price, Consensus and EPS Surprise
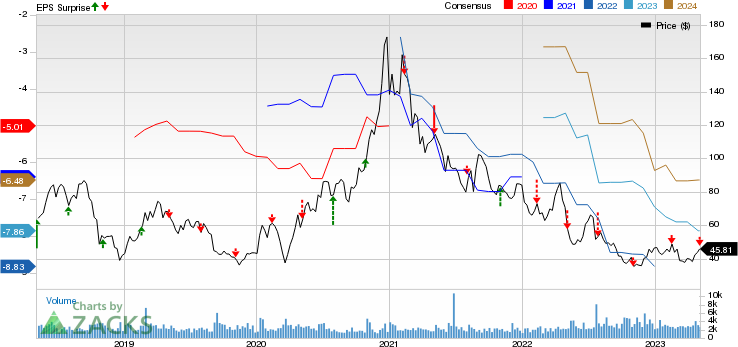
Ultragenyx Pharmaceutical Inc. price-consensus-eps-surprise-chart | Ultragenyx Pharmaceutical Inc. Quote
Zacks Rank & Stocks to Consider
Ultragenyx currently carries a Zacks Rank #3 (Hold).
Some better-ranked stocks in the biotech sector are Aptinyx APTX and 89BIO ETNB, both carrying a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
In the past 90 days, the Zacks Consensus Estimate for Aptinyx’s 2023 loss per share has narrowed from 77 cents to 47 cents. This year, shares of Aptinyx have fallen by 58.6%.
APTX beat estimates in each of the trailing four quarters, delivering an average earnings surprise of 6.56%.
In the past 90 days, the consensus estimate for 89BIO’s 2023 loss per share has narrowed from $2.59 to $2.29. This year, shares of 89BIO have increased by 26.9%.
ETNB beat estimates in three out of the trailing four quarters, missing the mark on one occasion, delivering an average earnings surprise of 12.55%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Regeneron Pharmaceuticals, Inc. (REGN) : Free Stock Analysis Report
Ultragenyx Pharmaceutical Inc. (RARE) : Free Stock Analysis Report
Aptinyx Inc. (APTX) : Free Stock Analysis Report
89BIO (ETNB) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 