Regeneron (REGN) Announces Positive Data on Oncology Candidate
Regeneron Pharmaceuticals, Inc. REGN announced positive new results from an ongoing phase I/II study on pipeline candidate REGN7075.
The phase I/II first-in-human, open-label study is evaluating REGN7075, in combination with oncology drug Libtayo (cemiplimab). The study is currently enrolling patients with metastatic and locally advanced solid tumors who have exhausted standard treatment options.
The trial includes an ongoing phase I dose-escalation portion and a phase II dose-expansion period. Patients first receive a weekly lead-in dose of REGN7075 monotherapy for three weeks to assess its safety and efficacy alone in the phase I dose-escalation portion. This is followed by treatment with combination therapy, wherein Libtayo is dosed once every three weeks and REGN7075 is dosed either every week or every three weeks.
Data from the dose-escalation portion of the trial showed that the investigational combination led to anti-tumor responses in patients with microsatellite stable colorectal cancer (MSS CRC). Among 94 patients treated as of data cutoff, 65% (n=61) had MSS CRC, of which 51 MSS CRC patients were treated at an active dose level.
Efficacy results among these 51 patients showed a 6% (n=3) overall response rate (ORR) and 29% (n=15) disease control rate. This included one complete response (CR), two partial responses (PR) and 12 patients with stable disease.
An ORR of 20% and a DCR of 80% were observed among the subset of 15 patients without liver metastases. Three patients had stable disease as of the data cutoff, and one patient achieved a PR following data cutoff among the subset of 36 patients with liver metastases.
The combination of REGN7075 and Libtayo showed an acceptable safety profile as assessed in 84 patients across multiple solid tumor types at a variety of doses of REGN7075. The maximum tolerated dose was not reached. Treatment-emergent adverse events of any grade occurred in 98% of patients.
The dose escalation portion of the trial across multiple solid tumor types, including non-small cell lung cancer, colorectal cancer, head and neck cancer and other tumor types, is currently ongoing. In addition, expansion cohorts in several tumor types have also been initiated.
These results will be shared during an oral session at the American Society of Clinical Oncology 2024 Annual Meeting in Chicago.
Libtayo is a fully human monoclonal antibody indicated in certain patients with advanced basal cell carcinoma, advanced cutaneous squamous cell carcinoma (CSCC) and advanced non-small cell lung cancer, as well as in advanced cervical cancer in many countries.
Regeneron is currently looking to expand its portfolio beyond Libtayo. Its oncology pipeline includes other candidates like linvoseltamab, odronextamab and fianlimab.
Regeneron’s shares have risen 11.7% year to date against the industry’s decline of 5.9%.
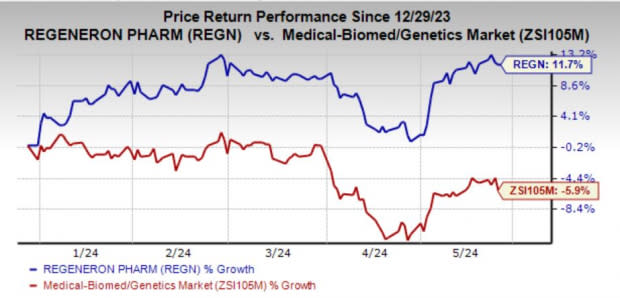
Image Source: Zacks Investment Research
The FDA accepted Regeneron’s biologics license application (BLA) seeking accelerated approval for linvoseltamab, a bispecific antibody targeting BCMA and CD3, to treat adult patients with relapsed/refractory (R/R) multiple myeloma that has progressed after at least three prior therapies. The BLA was granted priority review with a target action date of Aug 22, 2024. A phase III confirmatory trial is currently enrolling patients. A regulatory application is also under review in the EU.
However, Regeneron’s efforts to expand its oncology portfolio suffered a setback when the FDA issued Complete Response Letters (CRLs) for the BLA for odronextamab in R/R follicular lymphoma (FL) and R/R diffuse large B-cell lymphoma.
Regeneron has also initiated a phase II/III study of the combination of fianlimab, an antibody to LAG-3, and Libtayo in first-line metastatic melanoma. This study is enrolling faster than expected and will be conducted solely as a phase III study with the final analysis to be reported during 2025.
The successful development of its oncology candidates will be a major boost for REGN as it will reduce the company’s dependence on lead drug Eylea.
Eylea sales have been under pressure due to competition from Roche’s RHHBY Vabysmo.
To counter the decline in Eylea sales, Regeneron developed a higher dose of the drug. The initial uptake of Eylea HD is encouraging as Eylea patients transition to the higher dose.
However, it may be a while before the higher dose can compensate for the loss in Eylea sales.
The uptake of Vabysmo has been outstanding. RHHBY designed Vabysmo to block pathways involving Ang-2 and VEGF-A. The European Commission also approved Vabysmo for these indications.
Regeneron co-developed Eylea with Bayer AG BAYRY. It records net product sales of Eylea in the United States and Bayer records net product sales of the drug outside the country. Regeneron records its share of profit/loss in connection with the sales of Eylea outside the United States.
Zacks Rank & a Stock to Consider
Regeneron currently carries a Zacks Rank #3 (Hold).
A better-ranked stock in the biotech sector is ALX Oncology Holdings ALXO, which currently carries a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
In the past 30 days, the Zacks Consensus Estimate for ALX Oncology’s 2024 loss per share has narrowed from $3.33 to $2.89. During the same period, the consensus estimate for 2025 loss per share has narrowed from $2.85 to $2.73.
ALX Oncology beat on earnings in two of the trailing four quarters and missed the mark in the other two, delivering an average negative surprise of 8.83%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Regeneron Pharmaceuticals, Inc. (REGN) : Free Stock Analysis Report
Roche Holding AG (RHHBY) : Free Stock Analysis Report
Bayer Aktiengesellschaft (BAYRY) : Free Stock Analysis Report
ALX Oncology Holdings Inc. (ALXO) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 