QIAGEN (QGEN) Develops Test for Research and CDx Application
QIAGEN N.V. QGEN, together with Myriad Genetics MYGN, will develop a globally distributable kit-based test for analyzing Homologous Recombination Deficiency (HRD) status. The endeavor expands upon the two companies' recently-announced master collaboration agreement.
The next-generation sequencing test aims to aid personalized medicine research in solid tumor types, including ovarian cancer, and improve decentralized testing capabilities once a regulated product is developed.
The recent development will bolster QIAGEN’s Molecular Diagnostics business.
More on New Test Development
QIAGEN signed master collaboration agreements to develop and commercialize companion diagnostics with over 30 global pharmaceutical and biotech companies, resulting in a deep pipeline that advances precision medicine in a variety of disease indications by tailoring a patient's treatment to the genetic profile identified by companion diagnostics testing.
Myriad Genetics has given testing support for hundreds of clinical trials and gained 10 companion diagnostic approvals from the FDA and PMDA. QIAGEN hopes that this partnership will promote the expansion of Myriad Genetics’ oncology products portfolio.
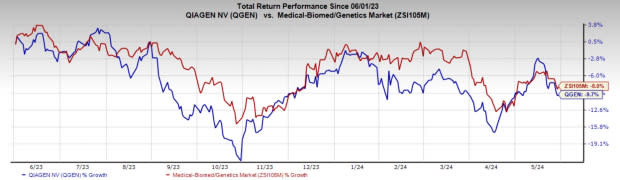
Image Source: Zacks Investment Research
The test will be based on QIAGEN's QIAseq xHYB technology, QIAGEN Digital Insight solutions, which generate a sample to insight HRD solutions and Myriad Genetics’ unique FDA-approved MyChoice CDx, a single-site PMA-approved centralized testing service for assessing HRD in specific tumors.
QIAGEN will oversee the development and distribution of the kit-based HRD test outside of the United States. The IP license allows QIAGEN to engage with pharmaceutical partners to develop an IVD-validated test for use as a companion diagnostic outside of the United States. The combined regulatory experience of QIAGEN and Myriad Genetics allows for smooth compliance and integration in clinical and companion diagnostic applications.
Strategic Efforts
QIAGEN's collaboration with Myriad Genetics aims to expand the availability of HRD tests, allowing a growing number of cancer patients to benefit from personalized therapy. QIAGEN anticipates that by introducing a distributable HRD test, they will be able to reduce the time required for therapeutic decisions, lower associated costs and shorter turnaround times when compared to outsourced testing, eventually benefiting patients.
By expanding the global reach and simplicity of access to Myriad Genetics’ gold-standard HRD-testing technology, QIAGEN hopes to contribute to the widespread and broader clinical use of HRD testing. This milestone highlights what the QIAGEN-Myriad Genetics collaboration can offer pharmaceutical partners, proprietary content, cutting-edge assay platforms, clinical trial execution and global CDx product distribution.
Industry Prospects
Per the Grand View Research report, the global PARP inhibitor biomarkers market size was estimated at $904.10 million in 2023 and is projected to witness a CAGR of 8.54% from 2024 to 2030. The PARP (poly-ADP ribose polymerase) inhibitor biomarkers market growth is attributed to the increasing prevalence of breast cancer, increasing advancements in genetic technologies and rising expenditures for oncology treatment.
Recent Developments
In May 2024, QIAGEN secured FDA clearance for the QIAstat-Dx Respiratory Panel Plus syndromic test. Previously authorized under the FDA Emergency Use Authorization (‘EUA’) as the QIAstat-Dx Respiratory SARS-CoV-2 Panel, the test is designed to support clinical decision-making in diagnosing upper respiratory infections and covers 21 viral and bacterial targets.
In March 2024, QIAGEN launched the QIAstat-Dx Analyzer 2.0, including the Software 1.6 upgrade, a significant enhancement to the widely-used QIAstat-Dx Analyzer 1.0 for fast and cost-effective diagnosis of complex syndromes. The upgraded diagnostic system introduces the Remote Results Application feature, which is still unique in the syndromic testing space.
Price Performance
In the past year, QGEN’s shares have dropped 9.7% compared with the industry’s 8% fall.
Zacks Rank and Key Picks
QIAGEN currently carries a Zacks Rank #3 (Hold).
Some better-ranked stocks from the broader medical space are Medpace MEDP and ResMed RMD.
Medpace, sporting a Zacks Rank #1 (Strong Buy), reported first-quarter 2024 earnings per share (EPS) of $3.20, beating the Zacks Consensus Estimate by 30.6%. Revenues of $511 million improved 17.7% from last year’s comparable figure. You can see the complete list of today’s Zacks #1 Rank stocks here.
Medpace has an estimated 2024 earnings growth rate of 26.5% compared with the industry’s 12.3%. The company’s earnings surpassed estimates in each of the trailing four quarters, the average being 12.8%.
ResMed, sporting a Zacks Rank #1, reported first-quarter 2024 EPS of $2.13, which topped the Zacks Consensus Estimate by 10.9%. Revenues of $1.20 billion surpassed the Zacks Consensus Estimate by 1.9%.
RMD has an estimated fiscal 2024 earnings growth rate of 17.9% compared to the industry’s 15.7%. In each of the trailing four quarters, the company delivered an average earnings surprise of 2.8%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
ResMed Inc. (RMD) : Free Stock Analysis Report
Myriad Genetics, Inc. (MYGN) : Free Stock Analysis Report
QIAGEN N.V. (QGEN) : Free Stock Analysis Report
Medpace Holdings, Inc. (MEDP) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 