Pharma Stock Roundup: J&J New Buyback Plan, CHMP Nod to PFE's BA.4, BA.5 Omicron Jab
This week, J&J JNJ announced plans to buy back $5 billion worth of shares and reaffirmed its profit outlook for 2022. Pfizer PFE and its partner BioNTech BNTX received a positive recommendation from the European Medicines Agency’s (“EMA”) Committee for Medicinal Products for Human Use (“CHMP”) for authorizing their Omicron BA.4 and BA.5-adapted COVID booster vaccine. The CHMP also recommended granting marketing approval to two of AstraZeneca’s AZN products, COVID-19 antibody cocktail drug, Evusheld and its investigational RSV vaccine, Beyfortus (nirsevimab)
Recap of the Week’s Most Important Stories
CHMP Nod to Pfizer’s Omicron BA.4/BA.5 Booster: The CHMP recommended granting conditional marketing authorization to Pfizer/BioNTech’s Omicron BA.4/BA.5-adapted bivalent vaccine. The recommendation is for use in individuals aged 12 years and older. The European Commission is expected to give its decision in a few days’ time. Pfizer/BioNTech expect the Omicron BA.4/BA.5 booster to be available before winter sets in. The FDA authorized the Omicron BA.4/BA.5-adapted booster on Aug 31.
The FDA accepted and granted standard review to Pfizer’s new drug application, seeking approval of ritlecitinib, its investigational JAK3 inhibitor for severe alopecia areata. The FDA’s decision is expected in the second quarter of 2023. The European Medicines Agency (EMA) also accepted ritlecitinib’s marketing authorization application. The EMA’s decision is expected in the fourth quarter of 2023. The regulatory applications were based on data from the phase IIb/III ALLEGRO study on ritlecitinib for severe alopecia areata, which showed that treatment with ritlecitinib led to significant scalp hair regrowth versus placebo.
Pfizer announced that the first participants have been dosed in a phase III efficacy study to evaluate a single dose quadrivalent mRNA-based influenza vaccine in healthy adults. The study will enroll 25,000 U.S. adults 18 years and older.
Pfizer’s phase III study, evaluating its investigational pentavalent meningococcal vaccine (MenABCWY) in healthy individuals 10 through 25 years of age, met all primary and secondary endpoints. In the study, the vaccine candidate was well tolerated with an acceptable safety profile. The protection provided by two doses of MenABCWY was non-inferior to licensed vaccines (two doses of Trumenba + one dose of Menveo) for the five meningococcal serogroups that cause the majority of invasive meningococcal disease: serogroups A, B, C, W and Y.
Based on this data, Pfizer plans to submit a regulatory application seeking approval for the pentavalent vaccine candidate in the fourth quarter. A pentavalent vaccine, if approved, can simplify what is currently a complex meningococcal vaccination schedule in the country
J&J’s New $5B Stock Buyback Plan: J&J’s board of directors authorized a new share buyback plan worth $5 billion. The buyback program has no time limit and may be suspended or discontinued at any time.
The company also reaffirmed its full-year sales and profit targets. Adjusted earnings per share are expected to be in the range of $10.15-$10.35. Operational constant-currency sales are expected to increase in the range of 6.5%-7.5%.
CHMP Recommends AstraZeneca’s Evusheld and Nirsevimab: AstraZeneca announced that the CHMP recommended marketing authorization of its COVID-19 antibody cocktail drug, Evusheld in the European Union (EU). The recommendation was based on the TACKLE phase III treatment data, showing reduced risk of severe COVID-19 or death.
The CHMP also gave a positive opinion recommending marketing authorization of nirsevimab for the prevention of respiratory syncytial virus (RSV) lower respiratory tract disease in newborns and infants during their first RSV season. The vaccine is expected to be marketed by the trade name of Beyfortus. The CHMP’s positive opinion was based on data from the MELODY and other phase IIb studies.
AstraZeneca announced that a phase III study called ALPHA, evaluating danicopan (ALXN2040) for treating patients with paroxysmal nocturnal haemoglobinuria (PNH) who experience clinically significant extravascular haemolysis, met the primary endpoint and key secondary endpoints. Danicopan is an investigational, oral factor D inhibitor as an add-on therapy to AstraZeneca’s C5 inhibitors Ultomiris (ravulizumab) or Soliris.
The study’s primary endpoint was change in hemoglobin from baseline at 12 weeks while the key secondary endpoints included transfusion avoidance and change in Functional Assessment of Chronic Illness Therapy (FACIT) Fatigue score. The interim data showed that danicopan plus Ultomiris or Soliris led to statistically significant and clinically meaningful improvements in hemoglobin levels, transfusion avoidance and FACIT Fatigue scores from baseline compared to placebo plus Ultomiris or Soliris for this specific patient population.
The NYSE ARCA Pharmaceutical Index declined 0.8% in the last five trading sessions.
Large Cap Pharmaceuticals Industry 5YR % Return
Large Cap Pharmaceuticals Industry 5YR % Return
Here’s how the eight major stocks performed in the last five trading sessions.
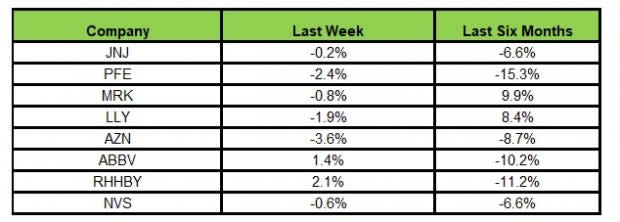
Image Source: Zacks Investment Research
In the last five trading sessions, Roche rose the most (2.1%) while AstraZeneca declined the most (3.6%).
In the past six months, Merck has gained the highest (9.9%) while Pfizer declined the most (15.3%).
(See the last pharma stock roundup here: FDA Nod to AZN’s Imfinzi for New Cancer & Other Updates)
What's Next in the Pharma World?
Watch out for regular pipeline and regulatory updates next week.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
AstraZeneca PLC (AZN) : Free Stock Analysis Report
Johnson & Johnson (JNJ) : Free Stock Analysis Report
Pfizer Inc. (PFE) : Free Stock Analysis Report
BioNTech SE Sponsored ADR (BNTX) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 