Pharma Stock Roundup: FDA Okays AZN's Capivasertib & MRK's Keytruda Expanded Use
This week, the FDA approved AstraZeneca’s AZN new breast cancer drug, Truqap (capivasertib) and the expanded use of Merck’s MRK blockbuster PD-LI inhibitor, Keytruda in a gastric cancer indication. Novo Nordisk NVO presented updated data from a cardiovascular outcomes study on its popular weight loss drug, Wegovy.
Recap of the Week’s Most Important Stories
FDA Approves AstraZeneca’s Truqap (capivasertib): The FDA granted approval to AstraZeneca’s capivasertib in combination with its other breast cancer drug Faslodex to treat HR-positive, HER2-negative metastatic breast cancer. Capivasertib will be marketed by the name of Truqap and has been approved for advanced breast cancer patients whose tumors have qualifying alterations in the PIK3CA, AKT1 or PTEN genes following recurrence or progression on or after an endocrine-based regimen. The approval was based on data from the CAPItello-291 phase III study in advanced HR-positive breast cancer. The FDA also approved a companion diagnostic test to detect the PIK3CA, AKT1 or PTEN alterations. Truqap in combination with Faslodex is also under review in Europe and several other countries.
A phase III study evaluating AstraZeneca’s Imfinzi for an early stage of non-small cell lung cancer (NSCLC) failed to reach statistical significance for the primary endpoint of progression-free survival.
The phase III study called PACIFIC-2 evaluated Imfinzi concurrently administered with platinum-based chemoradiotherapy (CRT) versus CRT alone in unresectable, stage III NSCLC.
At present, Imfinzi sequentially administered after platinum-based CRT is approved for the treatment of unresectable, stage III NSCLC based on data from the PACIFIC phase III study. The PACIFIC-2 study was initiated with the aim of concurrent Imfinzi administration with CRT for patients who progress or discontinue treatment during CRT and so become ineligible for the PACIFIC regimen.
FDA Approves Merck’s Keytruda for 7th Gastric Cancer Indication: The FDA approved Merck’s Keytruda plus fluoropyrimidine- and platinum-containing chemotherapy for the first-line treatment of patients with locally advanced unresectable or metastatic gastric or gastroesophageal junction (GEJ) adenocarcinoma. The approval was based on data from the KEYNOTE-859 study, which showed that Keytruda plus chemotherapy led to a significant improvement in overall survival in this patient group compared to chemotherapy alone. The approval marks the seventh one for a gastrointestinal cancer indication for the Keytruda-based regimen in the United States. A similar application is also under review in the EU.
Novo Nordisk’s Wegovy Shows Cardiovascular Benefits: At the American Heart Association annual Scientific Sessions (AHA) in Philadelphia, Novo Nordisk presented data from the SELECT study evaluating the effects of Wegovy in reducing cardiovascular risk in adults with overweight or obesity without diabetes. Previously reported top-line data from the study showed that Wegovy led to a statistically significant 20% risk reduction in major adverse cardiovascular events (“MACE”). The new data presented at AHA showed that the risk reductions in MACE were achieved regardless of age, gender, ethnicity and starting body mass index. The data also showed that the reductions in MACE risks began to appear soon after initiating Wegovy treatment, suggesting an effect of Wegovy beyond weight loss alone.
Novo Nordisk also announced plans to invest more than DKK 42 to expand existing manufacturing facilities in Kalundborg, Denmark.
The NYSE ARCA Pharmaceutical Index rose 0.42% in the last five trading sessions.
Large Cap Pharmaceuticals Industry 5YR % Return
Large Cap Pharmaceuticals Industry 5YR % Return
Here’s how the eight major stocks performed in the last five trading sessions.
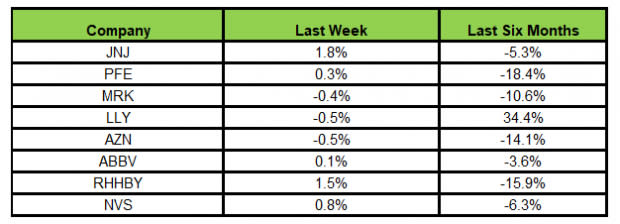
Image Source: Zacks Investment Research
In the last five trading sessions, J&J rose the most (1.8%), while AstraZeneca declined the most (0.5%).
In the past six months, Lilly has risen the most (34.4%), while Pfizer has declined the most (18.4%).
(See the last pharma stock roundup here: SNY, MRK, NVS Report Q3 Earnings, FDA Okays PFE, LLY Products)
What's Next in the Pharma World?
Watch out for pipeline and regulatory updates next week.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
AstraZeneca PLC (AZN) : Free Stock Analysis Report
Novo Nordisk A/S (NVO) : Free Stock Analysis Report
Merck & Co., Inc. (MRK) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 