Pfizer's (PFE) RSV Vaccine Abrysvo Gets Approval in EU
Pfizer PFE announced that the European Commission has approved its new vaccine, Abrysvo, for the prevention of respiratory syncytial virus (RSV) in infants through immunization of their pregnant mothers. In addition, Abrysvo was also approved for older adults in Europe.
In the EU, Abrysvo, Pfizer’s bivalent RSV prefusion F (RSVpreF) vaccine, is now approved for passive protection against lower respiratory tract disease (LRTD) caused by RSV in infants from birth through six months of age following maternal immunization during pregnancy. It is also approved for active immunization of individuals 60 years of age and older for the prevention of LRTD caused by RSV.
The EU approval was based on data from two pivotal phase III studies called RENOIR and MATISSE on the candidate.
Older adults, young infants and people with some chronic medical conditions are at maximum risk of getting LRTD-RSV disease. RSV is a contagious disease and a common cause of respiratory illness globally. The RSV disease causes approximately 245,000 hospital admissions in children and 270,000 hospitalizations among older adults yearly in Europe. The RSV vaccines are expected to reduce these infant and older adult hospitalizations this year.
Earlier this week the FDA approved Abrysvo to help protect infants through maternal immunization in the United States. Abrysvo was approved for older adults in the United States in May.In June, the U.S. Centers for Disease Control and Prevention’s (CDC) Advisory Committee on Immunization Practices also recommended Abrysvo for use in older adults aged 60 and older. The CDC’s recommendation for Abrysvo for use in pregnant mothers is awaited.
With the latest approvals, Pfizer’s vaccine has become the first maternal immunization vaccine to be approved to help protect infants at first breath through their first six months of life from RSV disease in the United States as well as the EU.
Pfizer is the only company with an RSV vaccine to help protect older adults and infants through maternal immunization in both the United States and EU.
Pfizer’s stock has declined 29.4% so far this year against an increase of 8.1% for the industry.
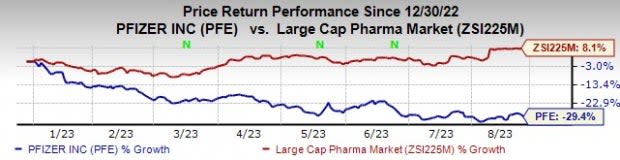
Image Source: Zacks Investment Research
In May, the FDA approved GSK’s GSK RSV vaccine, Arexvy, forthe prevention of LRTD caused by RSV in older adults aged 60+ years. This was the first RSV vaccine for older adults to be approved anywhere in the world. GSK’s Arexvy was approved in Europe in June 2023.
AstraZeneca AZN and Sanofi’s SNY RSV antibody called Beyfortus/nirsevimab was approved in the United States for protection against LRTI caused by RSV in newborns and infants in July. Beyfortus was approved in Europe in November 2022. The approval for Beyfortus was based on data from the MELODY phase III and other phase IIb studies conducted jointly by AstraZeneca and Sanofi.
Moderna has also developed an mRNA vaccine, mRNA-1345, targeting RSV in a phase III study, ConquerRSV, in older adults. The study met the primary efficacy endpoints, per top-line data announced in January. Based on these results, Moderna has filed for regulatory approvals of the RSV vaccine for older adults in several countries.
Pfizer currently has a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Pfizer Inc. Price and Consensus
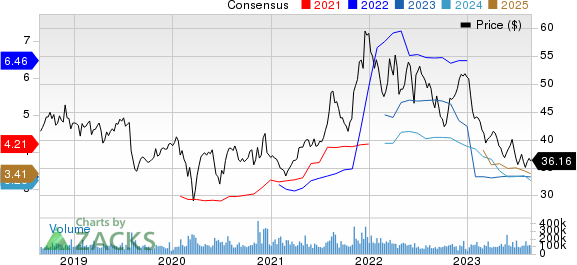
Pfizer Inc. price-consensus-chart | Pfizer Inc. Quote
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Sanofi (SNY) : Free Stock Analysis Report
AstraZeneca PLC (AZN) : Free Stock Analysis Report
GSK PLC Sponsored ADR (GSK) : Free Stock Analysis Report
Pfizer Inc. (PFE) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 