Pfizer (PFE), BioNTech's Updated COVID-19 Jab Gets CHMP Nod
Pfizer Inc. PFE and BioNTech SE BNTX announced that the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) has recommended marketing approval for their Omicron JN.1-adapted monovalent COVID-19 vaccine.
The CHMP has recommended the approval of Comirnaty JN.1, the Omicron JN.1-adapted monovalent COVID-19 vaccine, for active immunization against the COVID-19 virus in individuals aged six months and above.
The decision was based on the recommendation from the World Health Organization (WHO) Technical Advisory Group and the European Medicines Agency's Emergency Task Force (ETF). They recommended updating the COVID-19 vaccines to a monovalent (single strain) JN.1 composition for the 2024-2025 season.
The latest CHMP opinion was based on previous clinical, non-clinical and real-world evidence underlining the safety and efficacy of the COVID-19 vaccines developed by PFE and BNTX. It also includes manufacturing and pre-clinical data demonstrating that JN.1-adapted monovalent vaccines provide a substantially improved response against multiple Omicron JN.1 sublineages, including KP.2, KP.3 and other currently circulating sublineages.
The CHMP’s recommendation will now be reviewed by the European Commission and a final decision is expected shortly.
Shares of Pfizer have declined 3.5% year to date against the industry’s increase of 21.9%.
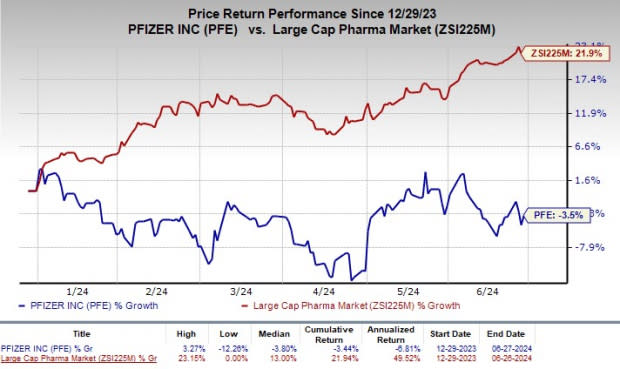
Image Source: Zacks Investment Research
Under the recent recommendation from the FDA, Pfizer and BioNTech are initiating rolling applications with the regulatory body, seeking approval of their Omicron KP.2-adapted monovalent COVID-19 vaccines for use in individuals aged six months and older.
In June 2024, another eminent vaccine maker, Moderna MRNA submitted an application to the FDA seeking approval of its Spikevax 2024-2025 formula, targeting the COVID-19 variant JN.1, the latest variant of the novel virus responsible for the 2020 pandemic.
MRNA’s regulatory filing was based on guidance from the FDA. The regulatory body advised other vaccine manufacturers, including Moderna, to update their respective COVID-19 vaccines to a monovalent (single strain) JN.1 composition for the 2024-2025 season.
Moderna simultaneously submitted regulatory applications worldwide to support registration and supply of the 2024-2025 formula of Spikevax in time for the upcoming vaccination season. Having already submitted an application for its Spikevax 2024-2025 formula in the United States, the company is expected to enjoy a first-mover advantage, subject to approval.
Earlier this month, Novavax NVAX, another vaccine maker, submitted an amendment to its Emergency Use Authorization to the FDA for its updated JN.1 COVID-19 vaccine (NVX-CoV2705) for use in individuals aged 12 years and older.
Compared with Pfizer and Moderna, Novavax was not able to reap the benefits of the pandemic due to a delayed launch of its COVID-19 vaccine.
The FDA has also pointed out that Pfizer/ BioNTech and Moderna’s vaccines, which are based on mRNA technology, can be developed more quickly when compared to Novavax’s protein-based COVID-19 vaccine.
We note that vaccine makers are suffering a heavy beatdown in product sales and market value as COVID-19 cases have significantly dropped compared with the last couple of years. Though rising COVID-19 infection cases could somewhat revive demand for vaccine boosters, the chances of driving revenues from vaccine sales look remote.
Zacks Rank
Pfizer currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Pfizer Inc. (PFE): Free Stock Analysis Report
Moderna, Inc. (MRNA): Free Stock Analysis Report
Novavax, Inc. (NVAX): Free Stock Analysis Report
BioNTech SE Sponsored ADR (BNTX): Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 