Medtronic's (MDT) OmniaSecure Lead Favored in the LEADR Trial
Medtronic plc MDT recently announced late-breaking data from the global Lead Evaluation for Defibrillation and Reliability (“LEADR”) Pivotal trial at Heart Rhythm 2024. The outcome showed that the investigational OmniaSecure defibrillation lead met its primary safety and effectiveness endpoints, exceeding prespecified performance goals.
The trial findings were simultaneously published in the Heart Rhythm journal. The development is likely to boost Medtronic’s Cardiac Rhythm Management business within the Cardiovascular Portfolio.
Significance of the OmniaSecure Defibrillation Lead
Implantable cardioverter defibrillators (ICDs) are crucial for preventing sudden cardiac death, but the thin wires connected to the ICD and threaded through the veins into the heart muscle remain the weakest point of the system. This is due to the harsh environment inside the human body where the lead must remain attached and yet flex with millions of heart contractions over a patient's lifetime. The large diameter (7-8 French) of existing defibrillation leads can contribute to venous occlusion or tricuspid valve complications.
Medtronic engineers designed the OmniaSecure defibrillation lead for patients where an extravascular defibrillator may not be appropriate. The lead is based on Medtronic’s SelectSecure Model 3830 pacing lead, which has offered safe and effective treatment to patients for more than 20 years. Remarkably, the Medtronic OmniaSecure lead is the world's smallest transvenous defibrillation lead (4.7 French, which is equivalent to the diameter of graphite in a wooden pencil). Presently, it is investigational worldwide and not yet approved for sale or distribution.
More on the Study Outcome
The LEADR Pivotal trial is a prospective, multicenter, single-arm, non-randomized, global clinical study that assessed the safety and effectiveness of the Medtronic OmniaSecure defibrillation lead when placed at the traditional right ventricle locations. The objective was to achieve defibrillation, sensing, pacing and cardioversion in patients at risk of sudden cardiac death. A total of 675 patients were enrolled in the study at 45 sites in 17 countries across North America, Europe, Asia and Australia.
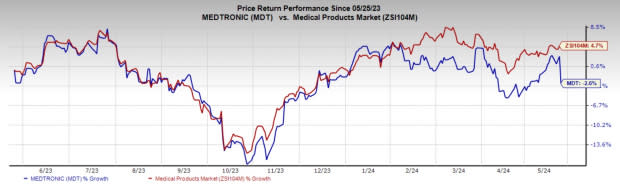
Image Source: Zacks Investment Research
The primary outcome revealed that the defibrillation testing conducted at device implantation in 119 patients was successful in 97.5% of cases, surpassing the prespecified efficacy goal of 88%. In six months, 97.1% (Kaplan-Meier estimate) of 657 patients with an implant attempt were free from lead-related major complications such as hospitalization, lead fracture, system revision or death. The study exceeded the prespecified safety performance goal of 90%. No lead-related major complications were observed between six and 12 months.
The lead demonstrated reliable performance with zero study lead fractures through the average follow-up months. Medtronic developed and validated an in-vitro model that accurately predicts lead reliability out to 10 years and then applied that model within the study. It predicted a fracture-free survival of 99.9% at two years for the investigational OmniaSecure lead. Moreover, the lead demonstrated a 97.9% implant success rate and stable electricals (R-wave, pacing capture threshold and pacing impedance) through 12 months.
Nearly 12% of patients in the study received appropriate therapy (shock or anti-tachycardia pacing) for dangerously fast ventricular arrhythmias. ATP terminated 74.9% of episodes, preventing a shock in 49 patients. Additional reliability model results from the LEADR study are expected to be presented by the company in the coming months. According to MDT’s chief medical officer of the Cardiac Rhythm Management business, the LEADR study results are an encouraging step forward in achieving the goal of an even more reliable defibrillation lead for patients.
Industry Prospects
Per a research report, the global defibrillator market was valued at $7.3 billion in 2023 and is expected to witness a CAGR of 8.1% by 2030.
Increasing product development activities, rising incidence of sudden cardiac arrest, growing awareness among the general public and supportive governments & healthcare initiatives are some of the key factors driving industry growth.
Other Developments in the Cardiovascular Portfolio
In addition to the LEADR results, Medtronic recently initiated the LEADR LBBAP study (Lead Evaluation for Defibrillation and Reliability in Left Bundle Branch Area Pacing), which is assessing the safety and efficacy of the OmniaSecure lead when placed at the Left Bundle Branch Area in patients eligible for an ICD or Left Bundle Branch-Optimized Cardiac Resynchronization Therapy (LOT-CRT).
Furthermore, the company’s Affera Mapping and Ablation System with Sphere-9 Catheter achieved endpoints for safety and efficacy in the SPHERE Per-AF study. Sphere-9 is an all-in-one pulsed field and radiofrequency ablation and high-density mapping catheter for the treatment of persistent atrial fibrillation.
Price Performance
In the past year, MDT shares have dropped 2.6% against the industry’s rise of 4.7%.
Zacks Rank and Key Picks
Medtronic currently carries a Zacks Rank #4 (Sell).
Some better-ranked stocks in the broader medical space are Hims & Hers Health HIMS, Medpace MEDP and ResMed RMD. While Hims & Hers Health and Medpace each sport a Zacks Rank #1 (Strong Buy), ResMed carries a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks Rank #1 stocks here.
Hims & Hers Heath stock has surged 75.3% in the past year. Estimates for the company’s earnings have risen from 10 cents to 18 cents in 2024 and from 23 cents to 32 cents in 2025 in the past 30 days.
HIMS’ earnings beat estimates in three of the trailing four quarters and missed in one, delivering an average surprise of 79.2%. In the last reported quarter, it posted an earnings surprise of a staggering 150%.
Estimates for Medpace’s 2024 earnings per share have moved up to $11.29 from $10.80 in the past 30 days. Shares of the company have surged 93.2% in the past year compared with the industry’s 8.7% growth.
MEDP’s earnings surpassed estimates in each of the trailing four quarters, the average surprise being 12.8%. In the last reported quarter, it delivered an average earnings surprise of 30.6%.
Estimates for ResMed’s fiscal 2024 earnings per share have moved to $7.64 from $7.43 in the past 30 days. Shares of the company have decreased 2.4% in the past year against the industry’s rise of 4.8%.
RMD’s earnings surpassed estimates in three of the trailing four quarters and missed in one, the average surprise being 2.8%. In the last reported quarter, it delivered an earnings surprise of 10.9%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Medtronic PLC (MDT) : Free Stock Analysis Report
ResMed Inc. (RMD) : Free Stock Analysis Report
Medpace Holdings, Inc. (MEDP) : Free Stock Analysis Report
Hims & Hers Health, Inc. (HIMS) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 