ImmunoGen (IMGN) Stock Surges 230% in a Month: Here's Why
ImmunoGen IMGN shares have rallied almost 228.8% in the past month, compared with the industry's 1.1% rise.
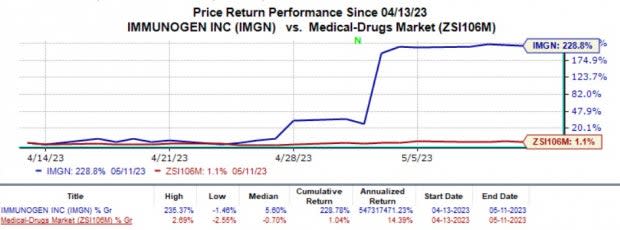
Image Source: Zacks Investment Research
A significant part of this upside can be attributed to the positive data from the phase III MIRASOL study evaluating Elahere for patients with folate receptor alpha (FRα)-positive platinum-resistant ovarian cancer (PROC).
ImmunoGen’s lead drug Elahere (mirvetuximab soravtansine) received an accelerated FDA approval in November 2022 to treat adults with FRα-positive, platinum-resistant epithelial ovarian, fallopian tube or primary peritoneal cancer, who have received one to three prior systemic treatment regimens.
The MIRASOL study is a confirmatory study for Elahere to get the accelerated approval converted to full approval.
The study showed that treatment with Elahere led to a statistically significant and clinically meaningful improvement in overall survival (OS) and progression-free survival (PFS), compared with investigator’s choice (IC) chemotherapy. The median OS was 16.46 months in the Elahere arm, compared with 12.75 months in the IC chemotherapy arm, representing a 33% reduction in the risk of death in the Elahere arm.
The median PFS in the Elahere arm was 5.62 months, compared with 3.98 months in the IC chemotherapy arm. The drug was also associated with a higher overall response rate of 42.3%, compared with 15.9% in the IC chemotherapy arm.
The safety profile of Elahere was also found to be favorable, with lower rates of adverse events compared with IC chemotherapy. It is the first drug to show an overall survival benefit in this patient population.
Shares of ImmunoGen were up more than 140% on May 3 as the company announced the positive data.
Based on the above data, the company plans to file a supplemental biologics license application with the FDA in the second half of 2023, seeking to convert the accelerated approval for Elahere to full approval. Management will also be filing a marketing authorization application to the European Medicines Agency in the same time frame, seeking approval of Elahere for FRα-high PROC.
The superiority of Elahere to chemotherapy in MIRASOL is expected to increase its use in off-label patient populations, such as platinum-sensitive and earlier lines of ovarian cancer patients with FRα expression.
The drug recorded its first full-quarter sales of $29.5 million in the first quarter of 2023, which was better than expected.
The company is currently evaluating Elahere in a phase II PICCOLO study and combination studies with bevacizumab and carboplatin in patients with platinum-sensitive ovarian cancer. Data from the PICCOLO study is expected before 2023-end.
ImmunoGen, Inc. Price and Consensus
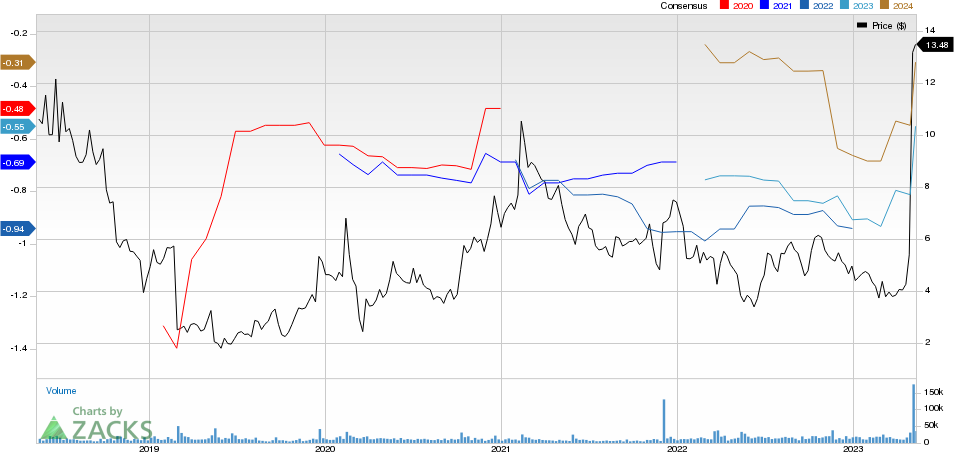
ImmunoGen, Inc. price-consensus-chart | ImmunoGen, Inc. Quote
Zacks Rank and Other Stocks to Consider
ImmunoGen currently carries a Zacks Rank #2 (Buy).
Some other top-ranked stocks in the same sector are Ocuphire Pharma OCUP, Allogene Therapeutics ALLO and Ligand Pharmaceuticals LGND. While Ocuphire Pharma sports a Zacks Rank #1 (Strong Buy), Allogene Therapeutics and Ligand Pharmaceuticals both carry a Zacks Rank of 2, at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
Loss per share estimates for Ocuphire Pharma has narrowed from 29 cents to 24 cents for 2023 and from 86 cents to 81 cents for 2024, in the past 60 days.
The company’s shares have surged 17.9% in the past month. Ocuphire’s earnings beat estimates in three of the last four quarters and missed the mark in one, the average surprise being 23.85%.
Loss per share estimates for Allogene have narrowed from $2.56 to $2.31 for 2023 and from $2.53 to $2.20 for 2024 in the past 60 days. Shares of ALLO have gained 23.3% in the past month.
Allogene’s earnings beat estimates in three of the last four quarters and missed the mark in one, the average surprise being 5.08%.
Earnings per share estimates for Ligand Pharmaceuticals have gone up from $4.15 to $4.16 for 2023 and from $4.28 to $4.58 for 2024 in the past 60 days. Shares of LGND have gained 2.5% in the past month.
Ligand Pharmaceuticals’ earnings outpaced estimates in two of the last four quarters and missed in the other two, the average surprise being 21.50%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
ImmunoGen, Inc. (IMGN) : Free Stock Analysis Report
Ligand Pharmaceuticals Incorporated (LGND) : Free Stock Analysis Report
Allogene Therapeutics, Inc. (ALLO) : Free Stock Analysis Report
Ocuphire Pharma, Inc. (OCUP) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 