Ideaya (IDYA) Up on Interim Data from Mid-Stage Eye Cancer Study
Shares of Ideaya Biosciences IDYA were up 30.7% during market hours on Sep 12, after the company announced positive interim data from its phase II clinical study. The study evaluated its solid tumor candidate, darovasertib, in combination with Pfizer’s PFE cancer therapy Xalkori (crizotinib) in first-line and any-line metastatic uveal melanoma (MUM) patients. The interim data showed a high percentage of patients with tumor shrinkage and encouraging partial responses in first-line and any-line MUM patients.
Ideaya’s shares have declined 48.9% in the year-to-date period compared with the industry’s return of 21.2%.
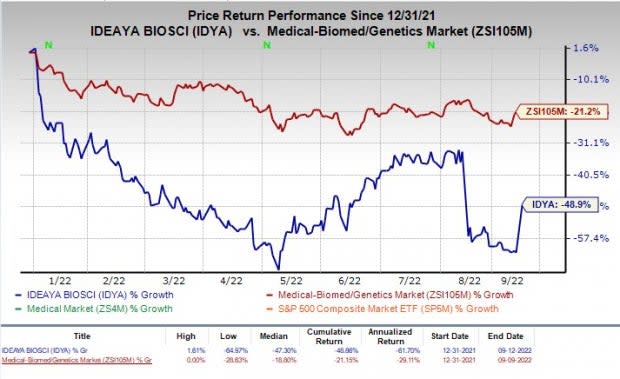
Image Source: Zacks Investment Research
MUM is a rare lethal melanoma arising from the melanocytes of the ciliary body, iris or choroid of the eye. It affects approximately 8,700 patients in the United States and Europe alone.
Darovasertib (IDE196) is a clinical-stage small molecule inhibitor of protein kinase C (PKC) being evaluated by Ideaya for genetically-defined cancers having GNAQ or GNA11 gene mutations, including MUM.
In March 2022, Ideaya entered into an additional clinical study collaboration and supply agreement with Pfizer to evaluate darovasertib, in combination with Xalkori, in a phase II study for treating MUM.
There are no approved therapies for cancers with GNAQ and GNA11 gene mutation. Therapies currently available for MUM have a relatively low objective response rate and short median progression free survival (PFS).
The results from the interim study evaluating darovasertib and Xalkori (crizotinib) combination in MUM demonstrated confirmed partial responses in four out of eight evaluable patients, equivalent to an overall response rate (ORR) of 50%, in first-line MUM patients. It also demonstrated 31% ORR in 11 of 35 evaluable any-line MUM patients.
The study also exhibited a shrinkage in tumors in 89% of any-line MUM patients enrolled in the study, while 83% exhibited a stable disease control rate (DCR).
Also, 15 (11 confirmed and 4 unconfirmed partial responses) out of 35 evaluable any-line MUM patients, i.e., 43% of patients, observed partial responses with greater than 30% tumor reduction.
Median Duration of Response (DOR) and Median Progression Free Survival (PFS) have not yet been reached in first-line MUM patients. PFS is more than five months in evaluable first-line MUM patients and five months for evaluable any-line MUM patients.
Ideaya intends to initiate a potential registration-enabling study evaluating darovasertib in combination with Xalkori (crizotinib) in the first quarter of 2023.
The interim data from the study are clinically significant and are built on the results reported in December 2021 from the preliminary darovasertib and crizotinib clinical combination study in heavily pre-treated MUM patients. However, the mid-stage study has a larger patient data set compared with its preliminary study.
Ideaya is also evaluating darovasertib as a monotherapy in phase I/II, in GNAQ and GNA11 solid tumors, including MUM and other solid tumor diseases such as cutaneous melanoma and skin melanoma. Darovasertib also achieved Orphan Drug designation for treating MUM from the FDA in April 2022.
The company is also looking to investigate darovasertib in other indications.
IDEAYA Biosciences, Inc. Price
IDEAYA Biosciences, Inc. price | IDEAYA Biosciences, Inc. Quote
Zacks Rank and Stocks to Consider
Ideaya Biosciences currently has a Zacks Rank #3 (Hold).
Some better stocks in the same sector are Immunocore IMCR and Sesen Bio SESN, each carrying a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 (Strong Buy) Rank stocks here.
Immuncore’s loss per share estimates for 2022 have narrowed from $2.44 to $1.34 in the past 30 days. The same for 2023 has narrowed from $2.76 to $1.78 in the same time frame.
Earnings of Immunocore missed estimates in two of the trailing four quarters and beat the same on the remaining two occasions. The average earnings surprise for IMCR is 33.28%.
Sesen Bio’s loss per share estimates for 2022 widened from 31 cents to 43 cents in the past 30 days. The same for 2023 has narrowed down from 10 cents to a cent in the same time frame.
Earnings of Sesen Bio missed estimates in all of the trailing four quarters. The average negative earnings surprise for SESN is 89.49%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Pfizer Inc. (PFE) : Free Stock Analysis Report
SESEN BIO, INC. (SESN) : Free Stock Analysis Report
IDEAYA Biosciences, Inc. (IDYA) : Free Stock Analysis Report
Immunocore Holdings PLC Sponsored ADR (IMCR) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 