Eli Lilly (LLY) Alzheimer's Drug Donanemab Gets FDA Panel Nod
An FDA committee assessing the safety and effectiveness of Eli Lilly and Company’s LLY Alzheimer's disease drug, donanemab, voted 11-0, thus unanimously recommending its approval.
The Peripheral and Central Nervous System Drugs Advisory Committee (PCNS) said that data from clinical studies supported the efficacy of donanemab for early symptomatic Alzheimer’s patients. The committee said the studies demonstrated substantial evidence of efficacy and clinically meaningful slowing of disease progression that was consistent across trials, populations, and endpoints. The data also demonstrated a greater benefit for those in the earlier stage of their disease. The committee said the safety profile of donanemab was well characterized. Overall, the committee voted in favor of the benefits of donanemab outweighing the risks, despite some safety concerns.
Lilly’s biologics license application (BLA) is seeking approval of donanemab for the treatment of patients with AD presenting mild cognitive impairment or mild dementia and with confirmed amyloid pathology.
Lilly’s stock was up 2.2% in after-hours trading on Jun 10. The stock has risen 48.4% year to date compared with an increase of 20.1% for the industry.
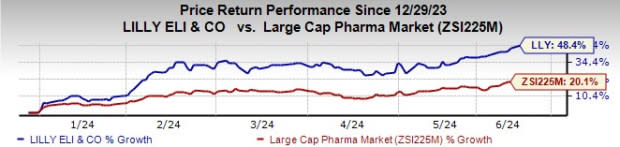
Image Source: Zacks Investment Research
Donanemab’s BLA was based on data from the TRAILBLAZER-ALZ 2 phase III study, which evaluated the drug in participants aged 60-85 years with early symptomatic Alzheimer's disease. Earlier data presented by Lilly from the study showed that treatment with donanemab significantly slowed cognitive and functional decline in early symptomatic Alzheimer's disease patients.
Almost 47% of the patients at an earlier stage of disease treated with donanemab had no clinical progression at 1 year compared with 29% on placebo. In patients with the earliest stage of the disease, an even greater benefit was observed, with a 60% slowing of decline compared to placebo. Treatment with donanemab slowed clinical decline by 35% compared to placebo.
Alzheimer's disease is a devastating neurodegenerative disorder characterized by the accumulation of tau tangles and amyloid beta (Aβ)plaques in the brain. Donanemab is an Aβ targeting therapy. Aβ is a protein that is said to be the primary cause of the cognitive decline associated with Alzheimer’s disease. However, anti-amyloid antibodies like donanemab can cause a brain swelling side effect called amyloid-related imaging abnormalities (ARIA). In clinical studies, ARIA-E (ARIA - edema/effusions) was observed in 24% and ARIA-H (ARIA-hemorrhage/hemosiderin deposition) in 31% of donanemab-treated patients. However, to Lilly’s advantage, the panel said such risks could be addressed by “appropriate labeling and management.”
Lilly had filed the BLA in mid-2023 and the FDA decision on donanemab was initially expected in the first quarter of 2024. However, in March 2024, the FDA said that a meeting of an advisory committee would be convened to discuss data from the TRAILBLAZER-ALZ 2 study to get more information about donanemab’s safety and effectiveness. The FDA is expected to give its decision later this year, which may or may not align with the PCNS recommendation.
Donanemab will be the second drug on the market to treat Alzheimer's disease if approved. Biogen BIIB and its Japan-based partner Eisai’s Leqembi was the first approved therapy that had shown a reduction in the rate of disease progression and slowed down cognitive and functional decline in adults with Alzheimer’s disease. Leqembi was granted full approval as a monthly intravenous therapy by the FDA for early Alzheimer’s disease in the United States in July 2023. The Centers for Medicare & Medicaid Services (“CMS”) has also granted broad reimbursement to Leqembi under Medicare plans. Leqembi is also approved in China and Japan. Regulatory applications seeking approval of Leqembi are under review in Europe.
Though the Leqembi launch is progressing slowly, Biogen believes it has the potential to generate blockbuster sales as there remains a massive unmet need for Alzheimer's disease. Biogen expects sales of Leqembi to start growing from 2024 with signs of acceleration seen in the first quarter.
Biogen/Eisai’s controversial medicine, Aduhelm, was approved by the FDA in 2021. However, in January 2024, Biogen discontinued the development and commercialization of Aduhelm as the drug failed to generate any significant sales due to a lack of access to Medicare beneficiaries.
Prothena Corporation PRTA also has antibody candidates targeting the Alzheimer’s disease indication in its pipeline. Prothena’s Alzheimer’s candidate is PRX012, a next-generation subcutaneous antibody targeting a key epitope at the N-terminus of Aβ. PRX012 is in early-stage development. The FDA has granted Fast Track Designation to PRX012 for the treatment of Alzheimer's disease. Prothena is also developing a dual Aβ-tau vaccine, PRX123, a potential prevention and treatment for Alzheimer's disease.
Lilly currently has a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Eli Lilly and Company Price and Consensus
Eli Lilly and Company price-consensus-chart | Eli Lilly and Company Quote
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Biogen Inc. (BIIB) : Free Stock Analysis Report
Eli Lilly and Company (LLY) : Free Stock Analysis Report
Prothena Corporation plc (PRTA) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 