Editas (EDIT) Pauses Eye Disease Study on EDIT-101, Stock Down
Editas Medicine, Inc. EDIT announced data from the phase I/II BRILLIANCE study evaluating its lead pipeline candidate, EDIT-101, for treating blindness due to Leber congenital amaurosis type 10 (LCA10).
The BRILLIANCE study included safety and efficacy data from all 14 patients (12 adults and two pediatric patients) who were being treated for LCA10.
Per the company, data from the study showed that three out of 14 subjects who were treated met a responder threshold, having experienced clinically meaningful improvements in best corrected visual acuity. These three patients demonstrated consistent improvements in two of the three additional endpoints: full field sensitivity test, visual function navigation course, or the visual function quality of life.
Overall, the study achieved proof of concept and identified a responder population. However, two of the three responders were homozygous for IVS26 mutation, as concluded after an examination of baseline characteristics of the treatment responder patients. However, according to Editas, LCA10 patients homozygous for CEP290 IVS26 mutation is a small patient population of 300 in the United States.
Given this small patient population, which is expected to respond to the therapy, Editas decided to pause enrollment in the BRILLIANCE study. EDIT decided not to proceed this program independently and actively seek a collaboration partner to continue the further development of EDIT-101. Treatment with EDIT-101 was tolerated with no ocular serious adverse effects or dose-limiting toxicities reported.
It remains to be seen whether Editas will eventually get a partner for developing EDIT-101 in the days ahead and what is the next path forward in case it does not get one.
Shares of Editas were down 10.1% on Thursday, following the announcement of the news. The stock has plunged 58.6% so far this year compared with the industry’s decline 19.6%.
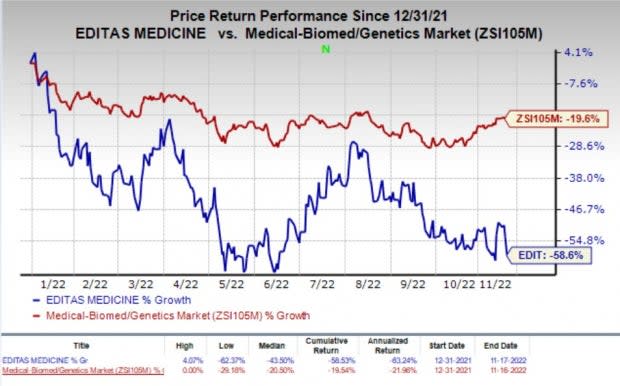
Image Source: Zacks Investment Research
EDIT-101, which employs CRISPR gene editing, is being developed to treat LCA10, a rare genetic illness that causes blindness. Currently, there is no effective treatment available for the given indication.
Editas is also developing other pipeline candidates, which can become a catalyst for the company in case the EDIT-101 program does not materialize.
The company is evaluating the safety and efficacy of its investigational gene-editing medicine, EDIT-301, for treating sickle cell disease.
Initial preliminary data from the RUBY study is expected to be presented by 2022-end.
Editas is also planning to begin the phase I/II EDITHAL study to evaluate EDIT-301 for treating transfusion-dependent beta thalassemia (“TDT”) patients. Dosing in the study is expected to begin shortly.
In May 2022, the FDA granted Orphan Drug designation to EDIT-301 for the treatment of TDT.
Zacks Rank & Stocks to Consider
Editas currently carries a Zacks Rank #3 (Hold). Some better-ranked stocks in the biotech sector are Immunocore Holdings plc IMCR, Angion Biomedica Corp. ANGN and Syndax Pharmaceuticals, Inc. SNDX, all sporting a Zacks Rank #1 (Strong Buy) at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
Loss per share estimates for Immunocore have narrowed 33.5% for 2022 and 33.3% for 2023 in the past 60 days.
Earnings of Immunocore surpassed estimates in three of the trailing four quarters and missed the same on the remaining occasion. IMCR witnessed an earnings surprise of 68.34% on average.
Loss per share estimates for Angion Biomedica have narrowed 6.7% for 2022 and 7.1% for 2023 in the past 60 days.
Earnings of Angion Biomedica surpassed estimates in three of the trailing four quarters and missed the mark on the other occasion. ANGN witnessed an earnings surprise of 66.42% on average.
Loss per share estimates for Syndax Pharmaceuticals have narrowed 4.9% for 2022 and 10.1% for 2023 in the past 60 days.
Earnings of Syndax Pharmaceuticals surpassed estimates in three of the trailing four quarters and met the same on the other occasion. SNDX witnessed an earnings surprise of 95.39% on average.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Angion Biomedica Corp. (ANGN) : Free Stock Analysis Report
Syndax Pharmaceuticals, Inc. (SNDX) : Free Stock Analysis Report
Editas Medicine, Inc. (EDIT) : Free Stock Analysis Report
Immunocore Holdings PLC Sponsored ADR (IMCR) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 