Corvus Pharma (CRVS) Stock Surges 69.2% in a Week: Here's Why
This week, shares of Corvus Pharmaceuticals CRVS shot up 69.2% against the industry’s 0.4% fall. The steep rise in the stock price of the company was observed after the company announced its recent business updates in the first quarter of 2023 earnings results, which showed the positive momentum built around CRVS’ pipeline development.
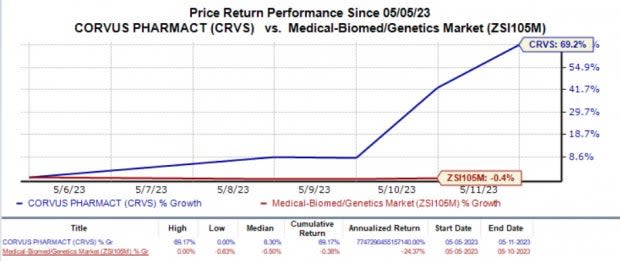
Image Source: Zacks Investment Research
Corvus Pharmaceuticals currently has no marketed product in its portfolio. The company is engaged in developing therapies alone as well as in partnership with other companies. CRVS’ lead candidate is CP-818, an oral ITK inhibitor, which is currently being evaluated as a single-agent therapy in a phase I/Ib study to treat patients with relapsed T cell lymphomas.
As of May 1, 2023, the company reported having enrolled 28 patients in the phase I/Ib study, receiving the maximum dose strength of 200 mg of CP-818. Out of the 28 patients enrolled, 19 were evaluable for tumor response. The company reported two complete responses (CR), one nodal CR and three partial responses (PR). Patients with PR are continuing to receive treatment. The remaining nine patients are on therapy, including five who have not had their initial tumor response evaluation.
Moreover, Corvus Pharmaceuticals reported that in patients with absolute lymphocyte count (ALC) above 900 per cubic milliliter of blood, objective responses(CR and PR) were observed in six of 13 patients with disease control (CR, PR and stable disease) in 11 of 13 patients. However, no observations were made in six patients with ALC below 900.
The median progression-free survival, for patients with ALC above 900 and ALC below 900, were 19.9 months and 2.1 months, respectively.
The company reportedly believes that the current enrollment rate in the phase I/Ib study of CP-818 will provide sufficient safety and preliminary efficacy data, forming the basis of its design of a potential registrational phase III study. Corvus intends to meet with the FDA in the third quarter of 2023 to discuss the parameters of a potential phase III study.
Apart from CP-818, Corvus and partner Kidney Cancer Research Consortium, reported enrolling patients in a phase Ib/II study evaluating ciforadenant, in combination with Yervoy (a CTLA-4 inhibitor) and Opdivo (a PD-1 inhibitor), as a potential first-line therapy for metastatic renal cell cancer. Notably, 60 patients are expected to be enrolled in the study, reporting top-line results by 2023-end.
Corvus and China-based Angel Pharmaceuticals also reported enrolling patients in a phase I/Ib study, evaluating mupadolimab in patients with non-small cell lung and head and neck squamous cell cancers. Patients will either receive mupadolimab as a monotherapy or in combination with Merck’s MRK Keytruda (pembrolizumab).
Merck’s blockbuster oncology drug, Keytruda, is approved for several types of cancer, accounting alone for around 40% of Merck’s pharmaceutical sales. Keytruda is presently approved to treat seven indications in earlier-stage cancers in the United States. Keytruda is continuously growing and expanding into new indications and markets globally.
Corvus Pharmaceuticals, Inc. Price and Consensus
Corvus Pharmaceuticals, Inc. price-consensus-chart | Corvus Pharmaceuticals, Inc. Quote
Zacks Rank and Other Stocks to Consider
Corvus Pharmaceuticals currently has a Zacks Rank #2 (Buy).
A few other stocks worth mentioning from the same industry are Allogene Therapeutics ALLO and Anixa Biosciences ANIX, both carrying a Zacks Rank #2 at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
In the past 90 days, the Zacks Consensus Estimate for Allogene Therapeutics’ 2023 loss per share has narrowed from $2.83 to $2.31. In the past week, shares of Allogene Therapeutics have fallen by 6.4%.
ALLO beat estimates in three of the trailing four quarters, missing the mark on one occasion, delivering an average earnings surprise of 5.08%.
In the past 90 days, the Zacks Consensus Estimate for Anixa Therapeutics’ 2023 loss per share has narrowed from 62 cents to 43 cents. In the past week, shares of Anixa Therapeutics have decreased by 5.6%.
ANIX beat estimates in each of the trailing four quarters, delivering an average earnings surprise of 24.04%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Merck & Co., Inc. (MRK) : Free Stock Analysis Report
Corvus Pharmaceuticals, Inc. (CRVS) : Free Stock Analysis Report
ANIXA BIOSCIENCES INC (ANIX) : Free Stock Analysis Report
Allogene Therapeutics, Inc. (ALLO) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 