Bristol Myers (BMY) Gets FDA Nod for Reblozyl Label Expansion
Bristol Myers Squibb BMY announced that the FDA has approved a label expansion of Reblozyl (luspatercept-aamt).
The drug is now approved for the treatment of anemia without previous erythropoiesis-stimulating agent use (ESA-naïve) in adult patients with very-low-to-intermediate-risk myelodysplastic syndromes (MDS) who may require regular red blood cell (RBC) transfusions. Hence, Reblozyl can now be used to address chronic anemia earlier in the treatment journey in a broader range of patients.
The label expansion of the drug in the first-line treatment of anemia for patients with lower-risk MDS was based on positive interim results from the late-stage COMMANDS trial, wherein Reblozyl demonstrated the superior efficacy of concurrent RBC transfusion independence and hemoglobin increase compared to epoetin alfa, an ESA, regardless of ring sideroblast status.
We remind investors that the drug is already indicated in the United States for the treatment of anemia in adult patients with beta thalassemia who require regular RBC transfusions. It is also approved for the treatment of anemia failing an erythropoiesis-stimulating agent and requiring two or more RBC units over eight weeks in adult patients with very-low-to-intermediate-risk MDS with ring sideroblasts (MDS-RS) or with myelodysplastic/myeloproliferative neoplasm with ring sideroblasts and thrombocytosis.
Bristol Myers is developing and commercializing Reblozyl through a global collaboration and North American co-promotion with Merck MRK, following Merck’s acquisition of Acceleron Pharma, Inc. in November 2021. As part of the collaboration, Merck receives milestone and royalty payments.
Sales of Reblozyl came in at $440 million in the first half of 2023. A broader label will boost sales.
Bristol Myers is currently in the transition mode as it shifts its mature product portfolio to new drugs. BMY shares have dropped 14.3% year to date compared with the industry's decline of 12.8%.
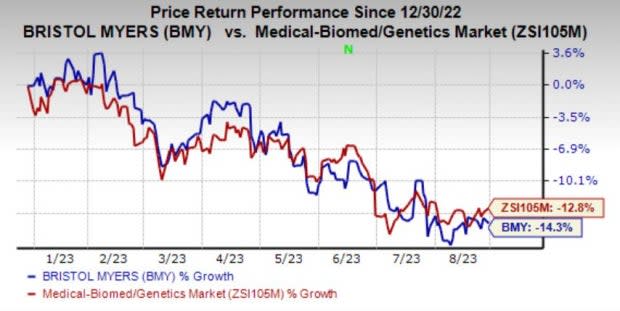
Image Source: Zacks Investment Research
The approval of new drugs and the label expansion of existing ones will enable Bristol Myers to diversify its product base and offset the slowdown in top-line growth, as Revlimid and Eliquis (in Canada and the U.K.) face generic competition.
Concurrently, BMY announced new long-term follow-up results from two phase III studies evaluating Camzyos (mavacamten), a first-in-class cardiac myosin inhibitor, in adult patients with symptomatic obstructive hypertrophic cardiomyopathy (HCM). Results from VALOR-HCM LTE (56 weeks) demonstrated that with a longer follow-up, Camzyos continued to reduce eligibility for invasive septal reduction therapy (SRT) at 56 weeks.
Data from the EXPLORER-LTE study (cumulative analysis up to 120 weeks) showed sustained improvements in left ventricular outflow tract obstruction, symptoms and NT-proBNP levels in patients with symptomatic obstructive HCM, with no new safety signals observed.
The approval of drugs like Opdualag, Reblozyl, Breyanzi and Sotyktu has added a new stream of revenues to BMY’s top line. Hence, the label expansion of these should fuel growth.
Zacks Rank and Stocks to Consider
Bristol Myers currently carries a Zacks Rank #3 (Hold).
A couple of better-ranked stocks in the biotech sector are Spero Therapeutics SPRO and Dynavax Technologies DVAX. While Spero sports a Zacks Rank #1 (Strong Buy) at present, Dynavax carries a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
Estimates for SPRO’s 2023 loss have narrowed by 39 cents to 51 cents per share in the past 30 days. Spero’s earnings beat estimates in all the trailing four quarters, the average surprise being 72.43%.
Estimates for DVAX’s 2023 loss have narrowed by 27 cents to 24 cents per share in the past 30 days. Dynavax has risen 36.2% in the year-to-date period. DVAX’s earnings beat estimates in two of the trailing four quarters and missed in the remaining two, the average surprise being 25.78%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Bristol Myers Squibb Company (BMY) : Free Stock Analysis Report
Dynavax Technologies Corporation (DVAX) : Free Stock Analysis Report
Merck & Co., Inc. (MRK) : Free Stock Analysis Report
Spero Therapeutics, Inc. (SPRO) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 