Bristol Myers (BMY) Gets EU Nod for Anemia Drug Reblozyl
Bristol Myers Squibb BMY announced that the European Commission (EU) has granted full marketing authorization to Reblozyl (luspatercept), for the treatments of anemia associated with non-transfusion-dependent (NTD) beta thalassemia in adult patients. Consequently, Reblozyl is now approved in all EU member states, Norway, Iceland and Liechtenstein. The drug currently is approved for treating anemia associated with transfusion-dependent beta thalassemia and transfusion-dependent lower-risk myelodysplastic syndromes.
In the United States, the FDA approved Reblozyl (luspatercept-aamt) for the treatment of anemia in adults with beta thalassemia, who require regular RBC transfusions, in November 2019. The drug’s label was also expanded for the treatment of anemia failing an erythropoiesis-stimulating agent and requiring two or more red blood cell (RBC) units more than eight weeks in adult patients with very low- to intermediate-risk myelodysplastic syndromes with ring sideroblasts or with myelodysplastic/myeloproliferative neoplasm with ring sideroblasts and thrombocytosis.
In the past year, BMY’s shares have increased slightly by 0.8% against the industry’s fall of 5.6%.
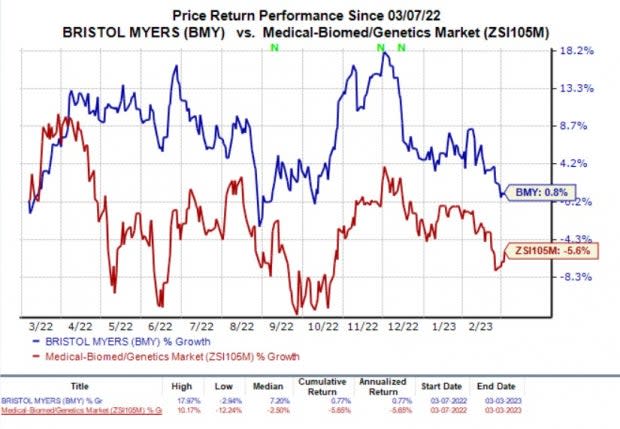
Image Source: Zacks Investment Research
The approval of Reblozyl in the EU was based on positive results from the phase II BEYOND study, which evaluated the efficacy and safety of Reblozyl in 145 adults with NTD beta thalassemia against treatment with a placebo. Results witnessed 74 of 96 (77.1%) patients treated with Reblozyl achieved the study’s primary endpoint, ≥1.0 g/dL mean Hb increase from baseline, versus 0 of 49 (0%) patients in the placebo arm (P<0.0001).
Beta thalassemia is an inherited blood disorder caused by a genetic defect in Hb, resulting in the production of fewer and less healthy RBCs. This often leads to severe anemia and is estimated to occur in 1 out of 100,000 people globally. There are limited treatment options for anemia associated with beta thalassemia which can cause organ damage. This represents a clear unmet medical need, globally, which management claims to have partially satisfied with the added approval in the EU.
Bristol Myers Squibb develops and commercializes Reblozyl through a global collaboration with medical powerhouse, Merck MRK. Merck acquired Acceleron Pharma in November 2021, following which, Merck collaborated with BMY for the development and commercialization of Reblozyl.
In a separate press release, BMY also announced the initiation of its pivotal phase III Librexia program, studying milvexian, an investigational oral factor XIa (FXIa) inhibitor (antithrombotic), for the prevention and treatment of major thrombotic conditions. This clinical program will be conducted in partnership with Janssen Pharmaceutical, a subsidiary of the pharma bigwig Johnson and Johnson JNJ.
This clinical program will collect data from nearly 50,000 patients across three indication-seeking studies, namely, Librexia STROKE, Librexia ACS and Librexia AF. The Librexia STROKE study will evaluate milvexian in combination with the standard-of-care antiplatelet therapy for stroke prevention in patients after an acute ischemic stroke or high-risk transient ischemic attack. Enrollment in the study has already commenced with the first patient enrolled.
The other two studies in the program, Librexia ACS, to evaluate event reduction in acute coronary syndromes in addition to standard-of-care antiplatelet therapy and the Librexia AF study, to investigate milvexian compared to apixaban in the prevention of stroke in patients with atrial fibrillation, are also scheduled to be initiated in the first half of 2023.
Bristol Myers Squibb Company Price and Consensus
Bristol Myers Squibb Company price-consensus-chart | Bristol Myers Squibb Company Quote
Zacks Rank and Stock to Consider
Bristol Myers Squibb currently has a Zacks Rank #3 (Hold).
A better-ranked stock in the same industry is Allogene Therapeutics, Inc. ALLO, carrying a Zacks Ranks #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
In the past 90 days, the estimate for Allogene’s 2022 loss per share has narrowed from $2.86 to $2.59. During the same period, the loss estimate per share for 2023 has narrowed from $2.85 to $2.47. In the past year, the shares of Allogene have fallen 25.3%.
ALLO’s earnings witnessed an average earnings surprise of 8.33%, beating all four estimates in the trailing four reported quarters.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Bristol Myers Squibb Company (BMY) : Free Stock Analysis Report
Johnson & Johnson (JNJ) : Free Stock Analysis Report
Merck & Co., Inc. (MRK) : Free Stock Analysis Report
Allogene Therapeutics, Inc. (ALLO) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 