Aurinia's (AUPH) Oral Lupus Drug Lupkynis Gets European Approval
Aurinia Pharmaceuticals AUPH announced that the European Commission (EC) granted marketing authorization to Lupkynis (voclosporin), an oral medicine for treating lupus nephritis (LN) in adults.
After the announcement of the European approval for Lupkynis, Aurinia’s shares were up 4.66% during market hours on Sep 19. However, the stock has declined 66.6% compared to the industry’s fall of 23.7% in the year-to-date period.
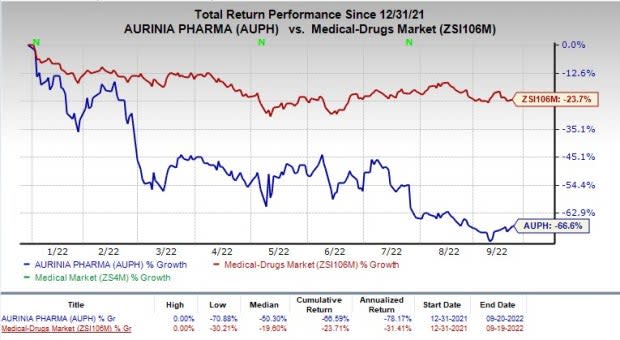
Image Source: Zacks Investment Research
The European marketing authorization comes after the company received a positive opinion in July 2022 from the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA), recommending approval of Lupkynis.
Lupkynis was approved by the FDA in the United States in January 2021.
Lupus Nephritis is a severe complication of the autoimmune disease, systemic lupus erythematosus (SLE). Almost half of the population who are diagnosed with SLE generally go on to develop lupus nephritis. If not controlled properly, LN can lead to permanent and irreversible renal tissue damage.
The European approval was based on data from two studies, namely the phase III AURORA 1 study and also the AURORA 2 combination study, both of which evaluated voclosporin in combination with mycophenolate mofetil (MMF) and low-dose corticosteroids. The study exhibited statistically superior complete renal response rates over 52 weeks in patients treated with voclosporin combination, compared to MMF and low-dose corticosteroids alone.
In December 2020, Aurinia collaborated with Japan’s Otsuka Pharmaceuticals for the development and commercialization of Lupkynis (voclosporin) for LN, in Europe and Japan, per a deal signed in December 2020.
Pursuant to this agreement, upon the recent EU approval for Lupkynis, Aurinia will receive a $30 million milestone payment from Otsuka to be recognized in the ongoing quarter. The company is also eligible to receive further payments related to additional regulatory and reimbursement milestones, low double-digit royalties on future net sales of the drug and revenues for the supply of product to Otsuka under a cost-plus arrangement.
Aurinia Pharmaceuticals Inc Price
Aurinia Pharmaceuticals Inc price | Aurinia Pharmaceuticals Inc Quote
Zacks Rank and Stocks to Consider
Aurinia currently has a Zacks Rank #3 (Hold).
Some better-ranked stock in the same sector is Acer Therapeutics ACER, Aerie Pharmaceuticals AERI and Soleno Therapeutics SLNO, each carrying a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 (Strong Buy) Rank stocks here.
Acer Therapeutics’ loss per share estimates for 2022 improved from $3.23 to $2.47 in the past 30 days. The same for 2023 has improved from $1.44 to $1.07 in the same time frame.
Earnings of Acer missed estimates in two of the trailing four quarters and beat the same on the remaining two occasions. The average negative earnings surprise for ACER is 106.16%.
Aerie Therapeutics’ loss per share estimates for 2022 improved from $3.47 to $2.47 in the past 30 days. The same for 2023 has improved from $1.49 to $1.07 in the same time frame.
Earnings of Aerie beat estimates in three of the trailing four quarters and missed the same in the remaining occasion. The average earnings surprise for AERI is 70.27%.
Soleno Therapeutics’ loss per share estimates for 2022 remained steady at $4.05 over the past 30 days. The same for 2023 has remained steady at $2.10 in the same time frame.
Earnings of Soleno missed estimates in three of the trailing four quarters and were in line on the remaining occasion. The average earnings surprise for SLNO is 11.40%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Aerie Pharmaceuticals, Inc. (AERI) : Free Stock Analysis Report
Aurinia Pharmaceuticals Inc (AUPH) : Free Stock Analysis Report
Soleno Therapeutics, Inc. (SLNO) : Free Stock Analysis Report
Acer Therapeutics Inc. (ACER) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 