AstraZeneca (AZN) Gets CHMP Nod for Evusheld to Treat COVID
AstraZeneca AZN announced that the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) has recommended marketing authorization of its antibody cocktail medicine, Evusheld in the European Union (EU) for the treatment of COVID-19.
Evusheld, which is a combination of tixagevimab and cilgavimab, has been recommended for the treatment of adults and adolescents (aged 12 years and older weighing at least 40 kgs) with COVID-19 who do not require supplemental oxygen and who are at increased risk of progressing to severe COVID-19. The recommendation was based on TACKLE phase III treatment data, which showed a reduced risk of severe COVID-19 or death on treatment with one intramuscular (IM) dose of Evusheld.
Evusheld is presently authorized for the prevention (pre-exposure prophylaxis) of COVID-19 in certain high-risk populations in Europe, the United States and some other countries. The medicine is not authorized for treating COVID-19. Only in Japan, it was recently authorized for pre-exposure prophylaxis as well as treatment of symptomatic disease caused by the SARS-CoV-2 infection.
This year so far, AstraZeneca’s shares have declined 0.3% compared with a decrease of 3.3% for the industry.
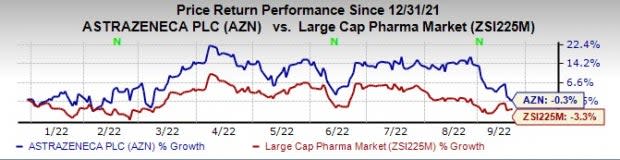
Image Source: Zacks Investment Research
The CHMP also recommended the marketing authorization of nirsevimab for the prevention of respiratory syncytial virus (RSV) lower respiratory tract disease in newborns and infants during their first RSV season. The vaccine is expected to be marketed by the trade name of Beyfortus. The CHMP’s positive opinion was based on data from the MELODY phase III, phase II/III and other phase IIb studies.
In a separate press release, AstraZeneca announced that a phase III study called ALPHA, evaluating danicopan (ALXN2040) for treating patients with paroxysmal nocturnal hemoglobinuria who experience clinically significant extravascular hemolysis, met the primary endpoint and key secondary endpoints. Danicopan is an investigational, oral factor D inhibitor, as an add-on to AstraZeneca’s C5 inhibitor therapy, Ultomiris (ravulizumab) or Soliris.
The study’s primary endpoint was the change in hemoglobin from baseline at 12 weeks while the key secondary endpoints included transfusion avoidance and change in Functional Assessment of Chronic Illness Therapy (FACIT) Fatigue score. The interim data showed that danicopan plus Ultomiris or Soliris led to statistically significant and clinically meaningful improvements in hemoglobin levels, transfusion avoidance and FACIT Fatigue scores from baseline compared to placebo plus Ultomiris or Soliris for this specific patient population.
Zacks Rank & Stocks to Consider
AstraZeneca currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Some better-ranked stocks in the same sector are Morphic MORF, Sesen Bio SESN and Agenus AGEN, all carrying a Zacks Rank #1 at present.
In the past 60 days, estimates for Morphic’s 2022 loss per share have narrowed from $3.47 to $1.80. Loss estimates for 2023 have narrowed from $3.96 to $3.62 during the same period. Shares of Morphic have lost 38.9% in the year-to-date period.
Earnings of Morphic beat estimates in three of the last four quarters and missed the mark just once. It came up with an earnings surprise of 48.29%, on average.
Estimates for Sesen Bio for 2023 have narrowed from a loss of 27 cents per share to a loss of 1 cent per share over the past 60 days. Shares of Sesen Bio have declined 16.% this year so far.
Earnings of Sesen Bio beat estimates in all the last four quarters, delivering a four-quarter surprise of 89.49%, on average.
Estimates for Agenus’ 2022 bottom line have narrowed from a loss of 89 cents to 70 cents in the past 60 days. Loss estimates for 2023 have narrowed from 64 cents per share to 60 cents per share over the same time frame. Agenus’ stock is down 27.3% in the year-to-date period.
Earnings of Agenus beat estimates in three of the last four quarters while missing in one. The stock delivered a four-quarter average negative surprise of 12.02%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
AstraZeneca PLC (AZN) : Free Stock Analysis Report
Agenus Inc. (AGEN) : Free Stock Analysis Report
SESEN BIO, INC. (SESN) : Free Stock Analysis Report
Morphic Holding, Inc. (MORF) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 