Amylyx (AMLX) Doses First Patient in Wolfram Syndrome Study
Amylyx Pharmaceuticals, Inc. AMLX announced that the first participant has been dosed in the HELIOS study of pipeline candidate AMX0035 (sodium phenylbutyrate [PB] and taurursodiol [TURSO]) for the treatment of Wolfram syndrome (WS). Shares were up 3.80% following the news.
HELIOS, a phase II study, is an exploratory open-label proof of biology study evaluating the effect of AMX0035 safety and tolerability and various measures of endocrinological, neurological and ophthalmologic function.
Top-line results from the study are expected in 2024.
WS, a rare progressive disease, is an autosomal recessive neurodegenerative disease characterized by childhood-onset diabetes, optic nerve atrophy and neurodegeneration.
The recently published preclinical data demonstrated initial proof-of-concept for the therapeutic development of AMX0035 (sodium phenylbutyrate and taurursodiol) in WS. Data also showed that a combination treatment of two chemicals may restore cellular functioning in a cellular model of WS.
We note that the FDA granted orphan drug designation to AMX0035 for the treatment of WS in November 2020.
In the year so far, the stock has plunged 19.5% against the industry’s 0.1% gain.
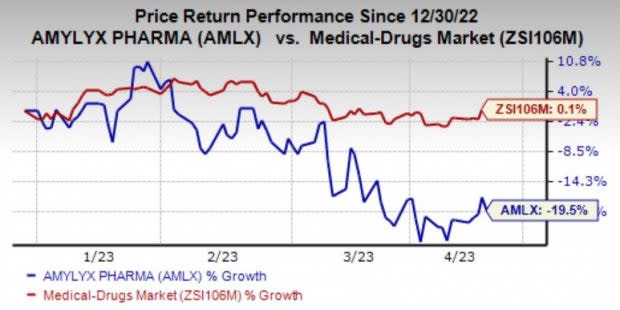
Image Source: Zacks Investment Research
Amylyx received a significant boost in 2022 as the FDA finally approved its drug for the treatment of adults with amyotrophic lateral sclerosis (ALS) under the brand name Relyvrio (sodium phenylbutyrate and taurursodiol).
The marketing authorization application (MAA) for the drug for the treatment of ALS is under review with the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP). The company now expects an opinion from CHMP by mid-year.
Zacks Rank & Stocks to Consider
Amylyx currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Some well-placed stocks in the overall healthcare sector are Novo Nordisk NVO and Ligand Therapeutics LGND, both sporting a Zacks Rank #1 at present.
In the past 30 days, estimates for Novo Nordisk’s 2023 earnings per share have risen from $4.20 to $4.43 and estimates for 2024 have gone up by 29 cents to $5.19.
Ligand’s earnings per share estimates for 2023 increased to $4.32 from $3.30 in the past 30 days. LGND beat earnings estimates in one of the last four reported quarters and missed in the remaining three.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Novo Nordisk A/S (NVO) : Free Stock Analysis Report
Ligand Pharmaceuticals Incorporated (LGND) : Free Stock Analysis Report
Amylyx Pharmaceuticals, Inc. (AMLX) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 