ACELYRIN (SLRN) Fails to Meet Goal in HS Study, Stock Down 54%
ACELYRIN, INC. SLRN, a clinical-stage company, announced dismal top-line results from Part B of its mid-late-stage study evaluating izokibep for the treatment of moderate-to-severe Hidradenitis Suppurativa (HS).
Izokibep is ACELYRIN’s small protein IL-17A inhibitor, which is currently being evaluated in late-stage studies to treat HS, psoriatic arthritis (PsA) and uveitis. The company is also planning to initiate an additional late-stage study on izokibep to treat axial spondyloarthritis.
Per the data readout, the Part B of the phase IIb/III study of izokibep failed to meet statistical significance in the primary endpoint of Hidradenitis Suppurativa Clinical Response (HiSCR75) at week 16. However, response rate data for the160 mg weekly dose of izokibep showed early HiSCR100 responses, which are supported by both pharmacokinetic exposures and HiSCR responses.
The study did not observe any safety concerns for izokibep and the drug was overall well-tolerated. Two cases of discontinuations, reported across the entire study, were due to injection site reactions.
The stock of the company nosedived 54.1% on Tuesday in response to the overall study failure news. Year to date, shares of ACELYRIN have plummeted 45.5% compared with the industry’s 12.8% fall.
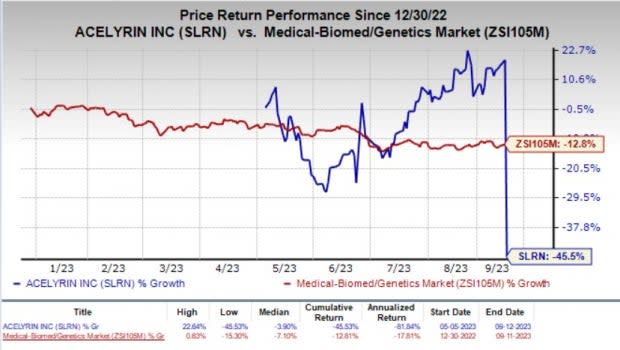
Image Source: Zacks Investment Research
The phase IIb/III study evaluated the safety and efficacy of izokibep 160 mg dosed weekly (QW) and every two weeks against treatment with placebo. The study enrolled 175 patients with moderate-to-severe HS.
ACELYRIN further stated that in the primary Non-Responder Imputation (NRI) analysis of Part B, statistical significance was impacted by patients with HiSCR75-100 discontinuing as early as week four unrelated to adverse events.
The company conducted a modified NRI analysis, adjusting for the high number of responders who discontinued in the QW arm. The modified NRI analysis data showed a high level of statistical significance, thereby reaffirming the impact of discontinuations on the magnitude and significance of response.
An independently conducted pre-planned interim analysis demonstrated HiSCR response rates of izokibep 160 mg QW, which is consistent with the Part A results. Early onset of HiSCR100 at week four, increasing through week 12 to 38% of patients, was also observed.
The company expects to report top-line data from its ongoing phase IIb/III study of izokibep in PsA by the end of the first quarter of 2024. Besides izokibep, ACELYRIN has another clinical-stage candidate, lonigutamab, which is currently in early-stage development, to treat thyroid eye disease (TED). The proof-of-concept data of lonigutamab from the TED study is also expected in first-quarter 2024.
Another player, MoonLake Immunotherapeutics MLTX, operates in the same market space as ACELYRIN. Moonlake is another clinical-stage company, which is currently developing its candidate, sonelokimab, in HS, PsA and psoriasis indications.
The rival company gained significantly from the HS study failure of ACELYRIN, as its stock climbed 10% on Tuesday, likely demonstrating positive investor expectations regarding key milestone events expected in the upcoming months.
MLTX’s sonelokimab is currently being evaluated in two phase II studies — MIRA in moderate-to-severe HS and ARGO in active PsA.
During the second quarter of 2023, MoonLake achieved a key milestone by reporting positive top-line 12-week data readout from its MIRA study evaluating the safety and efficacy of sonelokimab in patients with HS. The study met its primary endpoint by observing patients treated with both sonelokimab 120 mg and 240 mg achieving HiSCR75 against placebo at week 12 with statistical significance.
MoonLake is currently scheduled to announce MIRA 24-week data in HSin mid-October 2023.
In July 2023, MLTX reported having completed randomization of the target 200 patients ahead of schedule in its ARGO study in active PsA. ARGO 12-week data is expected in the first half of November 2023.
Moonlake’s psoriasis program evaluating sonelokimab is now phase III ready.
Sonelokimab is not yet approved by any regulatory body for use in any indication.
Zacks Rank and Stocks to Consider
ACELYRIN currently has a Zacks Rank #3 (Hold).
A few better-ranked stocks in the same industry are Dynavax Technologies DVAX and Corcept Therapeutics CORT, each carrying a Zacks Rank #2 (Buy) at present.
You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
In the past 30 days, the Zacks Consensus Estimate for Dynavax’s 2023 loss per share has remained constant at 24 cents. The estimate for Dynavax’s 2024 earnings per share is currently pegged at 2 cents. Year to date, shares of DVAX have risen by 27%.
DVAX’s earnings beat estimates in two of the trailing four quarters and missed the mark in the other two, delivering an average surprise of 25.78%.
In the past 30 days, the Zacks Consensus Estimate for Corcept’s 2023 earnings per share has gone up from 75 cents to 78 cents. The estimate for Corcept’s 2024 earnings per share has also improved from 81 cents to 83 cents. Year to date, shares of CORT have climbed 62.6%.
CORT’s earnings beat estimates in two of the trailing four quarters and missed the mark in the other two, delivering an average surprise of 6.99%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Dynavax Technologies Corporation (DVAX) : Free Stock Analysis Report
Corcept Therapeutics Incorporated (CORT) : Free Stock Analysis Report
MoonLake Immunotherapeutics (MLTX) : Free Stock Analysis Report
ACELYRIN, INC. (SLRN) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 