Verve (VERV) Pauses Enrollment in Cholesterol Study, Stock Falls
Verve Therapeutics VERV plunged 34.95% after the company announced updates from the Heart-1 phase Ib study on pipeline candidate VERVE-101.
VERVE-101 is a novel, investigational gene editing medicine designed to be a single-course treatment that permanently turns off the PCSK9 gene in the liver to reduce disease-driving low-density lipoprotein cholesterol (LDL-C).
The candidate is being evaluated in the phase Ib study with trial endpoints of safety and tolerability as well as changes in blood PCSK9 protein and low-density LDL-C levels in patients suffering from heterozygous familial hypercholesterolemia (HeFH), established atherosclerotic cardiovascular disease (ASCVD) and uncontrolled hypercholesterolemia.
A total of 13 participants have been dosed in the study. Among these, six were dosed at 0.45 mg/kg of VERVE-101. Data showed that the VERVE-101 demonstrated time-averaged LDL-C reductions ranging between 21% and 73%, and averaging 46% (as of a data cut-off date of Mar 18, 2024) for the first five participants in this cohort, with follow-up to at least 28 days.
In the two patients with the longest follow-up in the 0.45 mg/kg or 0.6 mg/kg cohorts, LDL-C lowering has been durable out to 270 days, with follow-up ongoing.
However, the sixth participant experienced a serious drug-related adverse event. This patient, treated in the 0.45 mg/kg cohort, experienced a grade 3 drug-induced transient increase in serum alanine aminotransferase as well as a serious adverse event of grade 3 drug-induced thrombocytopenia within the first four days after dosing.
Per the management, the abnormalities resolved fully within a few days and the participant did not experience any bleeding or other symptoms related to the laboratory abnormalities.
Consequently, the company has decided to pause enrollment in this study in consultation with the independent data and safety monitoring board. Verve is also investigating the laboratory abnormalities and will decide the path forward for the candidate based on the results. The results disappointed investors.
Verve’s shares plunged 37.8% in the past year compared with the industry's 0.7% decline.
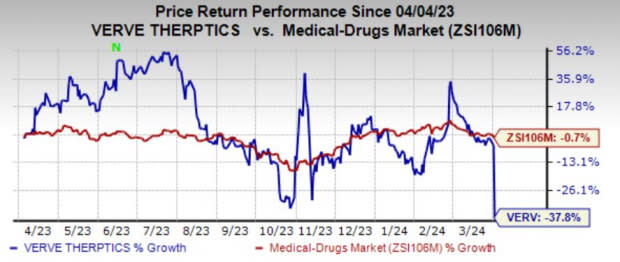
Image Source: Zacks Investment Research
Verve will now focus on the development of its other candidate, VERVE-102, and the initiation of the Heart-2 clinical trial.
While VERVE-102 uses the same base editor and guide RNA for PCSK9, it has a different lipid nanoparticle (LNP) delivery system than VERVE-101. Verve has received regulatory clearances for this study in the U.K. and Canada and plans to initiate the same in patients with HeFH or premature coronary artery disease in the second quarter of 2024.
The company also has another gene therapy, VERVE-201, in its pipeline. This is designed to permanently turn off the ANGPTL3 gene in the liver and is initially being developed for homozygous familial hypercholesterolemia (HoFH) and refractory hypercholesterolemia where patients still have high LDL-C, despite treatment with maximally tolerated standard of care therapies.
Verve entered into a research and collaboration agreement with Eli Lilly LLY in June 2023 for an exclusive, five-year worldwide research collaboration initially focused on advancing its discovery-stage in vivo 28 gene editing Lp(a) program.
Per the terms of the agreement, VERV is responsible for all research activities and phase I development of the initial target of interest. Eli Lilly, on the other hand, will assume the responsibility for further development, manufacturing and commercialization of the program.
Zacks Rank and Stocks to Consider
Verve currently has a Zacks Rank #3 (Hold). A couple of better-ranked stocks from the biotech sector are ADMA Biologics, Inc. ADMA and ANI Pharmaceuticals, Inc. ANIP, both sporting a Zacks Rank #1 (Strong Buy) at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
In the past 60 days, estimates for ADMA Biologics’ 2024 earnings per share (EPS) have improved from 22 to 30 cents. In the past year, shares of ADMA have surged 100%.
ADMA Biologics’ earnings beat estimates in three of the trailing four quarters and met once, delivering an average surprise of 85.00%.
In the past 60 days, estimates for ANI Pharmaceuticals’ 2024 EPS have improved from $4.06 to $4.43. In the past year, shares of ANIP have surged 76.9%.
ANI Pharmaceuticals’ earnings beat estimates in each of the trailing four quarters, delivering an average surprise of 109.06%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Eli Lilly and Company (LLY) : Free Stock Analysis Report
ANI Pharmaceuticals, Inc. (ANIP) : Free Stock Analysis Report
ADMA Biologics Inc (ADMA) : Free Stock Analysis Report
Verve Therapeutics, Inc. (VERV) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 