Valneva (VALN) Stock Down 66% in the Past 6 Months: Here's Why
Shares of Valneva VALN have dropped 66.2% in the past six months compared with the industry’s 42.6% decline.

Image Source: Zacks Investment Research
The downside was primarily caused by the revision in order for the supply of doses of VLA2001, the company’s inactivated COVID-19 vaccine.
Earlier this June, the European Commission (“EC”) granted marketing authorization to Valneva’s COVID-19 vaccine for use as a primary vaccine in adults aged 18 through 50 years in the European Union (EU). Following the approval, VLA2001 became the first COVID-19 vaccine to receive standard marketing authorization in Europe. The vaccine is also authorized for use in Bahrain, the United Arab Emirates and the United Kingdom.
The EC’s approval to VLA2001 was based on positive top-line data from the pivotal phase III Cov-Compare study, which evaluated VLA2001 against AstraZeneca’s AZN COVID vaccine, AZD1222. Data from this study demonstrated the superiority of VLA2001 over the AstraZeneca vaccine.
The study achieved both co-primary endpoints two weeks after the second dose of vaccination. VLA2001 produced superior neutralizing antibody titer levels to AstraZeneca’s AZD1222. Further, VLA2001 demonstrated the same effectiveness as the AstraZeneca vaccine in neutralizing antibody seroconversion rates by more than 95%.
Prior to securing the marketing approval in the EU, VALN also entered into an advance purchase agreement (“APA”) with the EC last year in November to supply up to 60 million doses of VLA2001 to the EU member states once the vaccine is granted marketing authorization.
One of the terms of this APA provided the EC with the right to terminate the agreement if VLA2001 does not receive marketing authorization in the EU by Apr 30, 2022. The EC decided to exercise this right in May 2022 and communicated its intent to terminate the APA. Following remediation discussions between the EC and VALN, the former approved an amendment to the APA, revising the supply to 1.25 million doses of VLA2001. The amended APA also allows EC to purchase another 1.25 million doses.
This reduced order received from the EC has resulted in management evaluating its COVID-19 program. Though this low order volume does not put pressure on its existing cash resources, the company has suspended manufacturing doses of VLA2001. Earlier this month, Valneva terminated its collaboration with IDT Biologika, its COVID-19 vaccine manufacturing partner. Management is also engaged in active discussions with potential partners on producing an updated version of its COVID-19 vaccine that targets new variants.
Apart from VLA2001, Valneva is evaluating other vaccines in its pipeline for Lyme disease and chikungunya.
Presently, the company is focused on developing VLA15, its vaccine candidate for Lyme disease. The candidate is being developed in collaboration with Pfizer PFE. Last month, Pfizer and Valneva initiated a phase III study to evaluate VLA15 in individuals aged five years and older. The initiation of the study has also triggered a milestone payment of $25 million payable by Pfizer to Valneva.
Earlier this June, Pfizer also bought an 8.1% stake in Valneva for $95 million. Valneva plans to use this money to support its contribution toward developing the vaccine.
Last month, Valneva announced that it has started a rolling BLA submission with the FDA, which seeks approval for the use of its single-shot chikungunya vaccine, VLA1553, in adults.
If approved, VLA1553 will also be the first vaccine for chikungunya and will be eligible for a priority review voucher. The vaccine has already been granted Breakthrough Designation therapy and Fast Track designations by the FDA.
Valneva SE Sponsored ADR Price
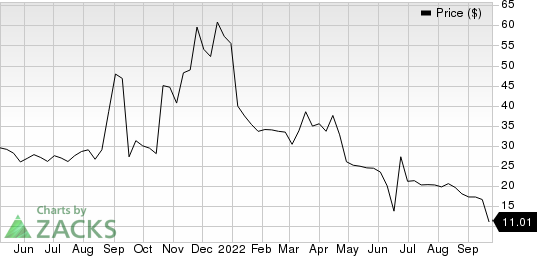
Valneva SE Sponsored ADR price | Valneva SE Sponsored ADR Quote
Zacks Rank & Stock to Consider
Valneva currently carries a Zacks Rank #3 (Hold). A better-ranked stock in the overall healthcare sector is Morphic MORF, which sports a Zacks Rank #1 (Strong Buy) at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
In the past 60 days, estimates for Morphic’s 2022 loss per share have narrowed from $3.38 to $1.80. Loss estimates for 2023 have narrowed from $3.91 to $3.62 during the same period. Shares of Morphic have lost 35.8% in the year-to-date period.
Earnings of Morphic beat estimates in three of the last four quarters and missed the mark just once, witnessing a surprise of 48.29%, on average. In the last reported quarter, MORF delivered an earnings surprise of 183.95%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
AstraZeneca PLC (AZN) : Free Stock Analysis Report
Pfizer Inc. (PFE) : Free Stock Analysis Report
Morphic Holding, Inc. (MORF) : Free Stock Analysis Report
Valneva SE Sponsored ADR (VALN) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 