Pfizer (PFE) Booster Dose Gets CDC Backing for Older Adults Only
On Thursday, an advisory committee of the Centers for Disease Control and Prevention (“CDC”) recommended that a booster or third dose of Pfizer Inc. PFE/ BioNTech SE’s BNTX mRNA-based COVID-19 vaccine, Comirnaty, should only be given to older adults aged 65 years and above, people living in long-term care facilities, and people aged 50 to 64 years with underlying medical conditions. However, the Advisory Committee on Immunization Practices was not in favor of giving the booster dose to younger people in certain high-risk professions, such as healthcare workers and teachers.
The latest CDC recommendation is not in line with the FDA’s emergency approval issued earlier on Wednesday. The FDA granted emergency use authorization to the booster dose of Comirnaty for individuals 65 years and older and for those in high-risk groups.
The FDA specified that the high-risk groups include individuals aged 16 to 64 who are at a high risk of severe COVID-19, and those who are highly exposed to the virus, making them vulnerable to COVID-related complications. The FDA said that the third jab should be given at least six months after the primary two-dose series.
However, in line with the recommendation of the FDA’s Vaccines and Related Biological Products Advisory Committee, last week, the FDA did not approve the booster dose of Comirnaty for the entire population (people 16 years of age and older) for which Pfizer/BioNTech were seeking approval.
Shares of Pfizer have rallied 20.1% so far this year compared with the industry’s rise of 8.6%.

Image Source: Zacks Investment Research
BioNTech’s stock has skyrocketed 333.1% year to date against a decrease of 0.7% for the industry.
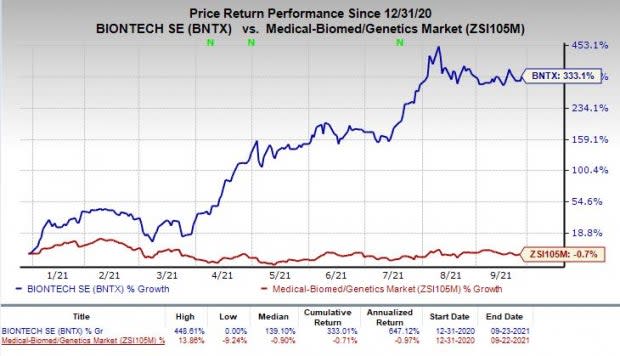
Image Source: Zacks Investment Research
Moderna, Inc. MRNA has also submitted an application for the authorization/approval for a booster dose of its COVID-19 vaccine, mRNA-1273, and has provided clinical data to support the efficacy of a booster dose, to the FDA.
The third doses of both mRNA-1273 and Comirnaty are presently authorized for use in immunocompromised individuals having a weakened immune system after six months of the second dose of the initial two-dose regimen.
Earlier this month, J&J JNJ presented additional data from the phase III ENSEMBLE study, which showed that a booster dose of its adenovirus-based single-shot vaccine, given 56 days after the first jab, led to 94% protection against mild-to-severe COVID-19 in the United States. J&J has submitted the latest additional data for the booster dose to the FDA.
Zacks Rank
Both Pfizer and BioNTech carry a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Johnson & Johnson (JNJ) : Free Stock Analysis Report
Pfizer Inc. (PFE) : Free Stock Analysis Report
Moderna, Inc. (MRNA) : Free Stock Analysis Report
BioNTech SE Sponsored ADR (BNTX) : Free Stock Analysis Report
To read this article on Zacks.com click here.

 Yahoo Finance
Yahoo Finance 