Novavax (NVAX) Applies to WHO for COVID-19 Vaccine Listing
Novavax NVAX announced that it has filed a regulatory application to the World Health Organization (“WHO”) for the emergency use listing of its COVID-19 vaccine, NVX-CoV2373.
The listing, if allowed, will enable the company to supply its COVID-19 vaccine to nations participating in the COVAX facility. The COVAX facility aims to provide equitable access to COVID vaccines across low- and lower-middle-income countries.
The company has partnered with Serum Institute of India (“SII”), the world’s largest vaccine producer, to jointly supply 1.1 billion doses of NVX-CoV2373 to countries participating through the COVAX facility.
Both Novavax and SII have already completed the submission of regulatory filings for Emergency Use Authorization (“EUA”) of NVX-CoV2373 in India, Indonesia, and the Philippines. The submission to WHO is based on the regulatory submission made to the Indian health regulator.
Novavax’s shares increased 10.9% on Sep 23, following the above news. In fact, the stock has rallied 130.7% so far this year in comparison with the industry’s 0.7% increase.

Image Source: Zacks Investment Research
Data from the phase III PREVENT-19 study, which evaluated NVX-CoV2373 in the United States and Mexico, were announced in June. The study met its primary endpoint by demonstrating an overall vaccine efficacy of 90.4%. The vaccine also provided 100% protection against moderate and severe coronavirus disease - a key secondary endpoint.
The company is also evaluating NVX-CoV2373 in another phase III study in the United Kingdom. Data from the study has demonstrated that the vaccine achieved an overall efficacy of 89.7%. It also provided an efficacy of 96.4% against the original COVID-19 strain and 86.3% against the Alpha variant of the virus.
Currently, the WHO has granted EUL to the COVID vaccines developed by AstraZeneca AZN, Moderna MRNA and Pfizer PFE/BioNTech that supplied millions of doses to the world. Earlier this week, Pfizer/BioNTech announced plans to provide the U.S. government with 500 million additional doses of their COVID vaccine at a not-for-profit price for donation to members of COVAX and the African Union.
Although Novavax is yet to file regulatory applications in the United States for its COVID vaccine, NVX-CoV2373 holds strong potential as there is a requirement for several billion doses across the world. The company plans to apply to the FDA for EUA for NVX-CoV2373 in fourth-quarter 2021.
Novavax, Inc. Price
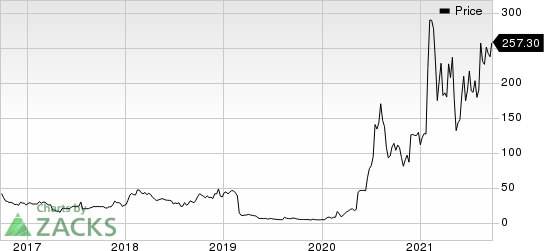
Novavax, Inc. price | Novavax, Inc. Quote
Zacks Rank
Novavax currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
AstraZeneca PLC (AZN) : Free Stock Analysis Report
Pfizer Inc. (PFE) : Free Stock Analysis Report
Moderna, Inc. (MRNA) : Free Stock Analysis Report
Novavax, Inc. (NVAX) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 