Genprex (GNPX) Expands SCLC Study of Key Candidate, Stock Up
Genprex, Inc.'s GNPX shares rose 9.5% after it announced the expansion of the Acclaim-3 clinical study of lead cancer candidate, Reqorsa, to additional trial sites.
Under collaboration with a large network of community-based oncology practices, Genprex is adding multiple clinical study sites for the early to mid-stage combination study of Reqorsa Immunogene Therapy (quaratusugene ozeplasmid) to treat patients with extensive-stage small cell lung cancer (ES-SCLC).
Reqorsa has been developed utilizing its proprietary ONCOPREX Delivery System technology that re-expresses tumor suppressor genes in cancers.
The study was previously expected to enroll ES-SCLC patients at three to five U.S. clinical sites. The figure has now doubled to approximately 10-15.
Per management, such an expansion is expected to accelerate patient enrollment by extending access to patients in remote areas. It is also expected to broaden the geographic reach of the innovative early-stage study to more ES-SCLC patients in need and potentially benefit those who have limited benefits from currently available therapies.
Year to date, shares of Genprex have plunged 66.2% against the industry’s 1.9% growth.
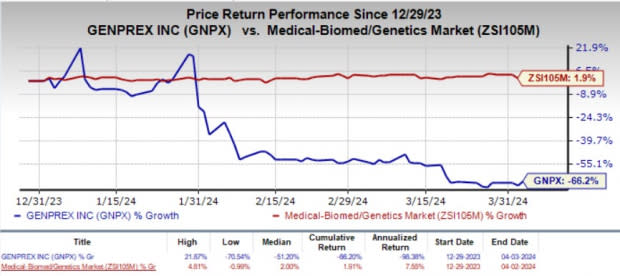
Image Source: Zacks Investment Research
The phase I/II Acclaim-3 study, evaluating the safety and efficacy of Reqorsa in combination with Roche’s RHHBY immuno-oncology drug, Tecentriq (atezolizumab), in ES-SCLC patients is planned to be conducted in two parts — a phase I dose-escalation portion and a phase II expansion portion receiving maintenance therapy.
The primary endpoint of the phase I dose-escalation portion of the study is to determine the maximum tolerated dose and recommend a dosage strength of Reqorsa for phase II development. On the other hand, the primary goal of the phase II portion is to determine the 18-week progression-free survival rate for the entire duration of the maintenance therapy with Reqorsa and Roche’s Tecentriq in patients with ES-SCLC.
The eligible patient population for the Acclaim-3 ES-SCLC study includes those who did not develop tumor progression after receiving Tecentriq and chemotherapy as standard initial treatment and are, therefore, eligible for maintenance therapy.
Genprex expects to complete the phase I portion of the Acclaim-3 study during the second half of 2024, followed by the initiation of the phase II expansion portion.
The Reqorsa/Tecentriq combination therapy currently enjoys the FDA’s Fast Track and Orphan Drug designations in the United States for the ES-SCLC indication. The Fast Track designation is designed to expedite the development and review of drugs to treat serious medical conditions and fulfill unmet curative needs. The Orphan Drug designation, on the other hand, will grant the combination therapy market exclusivity for a pre-specified period of time, subject to approval.
GENPREX Price and Consensus
GENPREX price-consensus-chart | GENPREX Quote
Roche’s Tecentriq is currently approved in the United States, EU and several other countries to treat some of the most aggressive and difficult-to-treat forms of cancer, including advanced lung cancer, urothelial cancer and breast cancer.
We remind the investors that Genprex is also currently evaluating Reqorsa in combination with AstraZeneca’s AZN Tagrisso (osimertinib) and Merck’s MRK Keytruda (pembrolizumab) in separate early to mid-stage studies (Acclaim-1 and Acclaim-2, respectively) to treat patients with late-stage non-small cell lung cancer (NSCLC).
Genprex believes that Reqorsa’s unique mechanism of action has the potential to provide improved treatment benefits when combined with marketed therapies for patients with NSCLC, SCLC and possibly other cancers.
AstraZeneca’s Tagrisso is already approved for the treatment of NSCLC. In 2023, Tagrisso sales accounted for 13% of AZN’s total revenues.
Keytruda, an anti-PD-1 therapy, is Merck’s blockbuster oncology drug and is approved for several types of cancers, accounting alone for around 45% of Merck’s pharmaceutical sales.
Merck’s Keytruda is presently approved to treat eight indications in earlier-stage cancers in the United States. Keytruda is continuously growing and expanding into new indications and markets globally, bolstering MRK’s position in the oncology market.
Zacks Rank and Stocks to Consider
Genprex currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
AstraZeneca PLC (AZN) : Free Stock Analysis Report
Roche Holding AG (RHHBY) : Free Stock Analysis Report
Merck & Co., Inc. (MRK) : Free Stock Analysis Report
GENPREX (GNPX) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 