Galapagos (GLPG) Concludes Enrollment in Crohn's Disease Study
Galapagos NV. GLPG announced that it has completed patient enrollment in the late-stage DIVERSITY study.
The study is evaluating the efficacy and safety of filgotinib, a JAK1 preferential inhibitor, in the induction and maintenance of remission in patients with Crohn’s Disease (CD).
The study enrolled 1,374 patients with moderately-to-severely active CD including biologic-naïve and biologic-experienced patients. The study evaluates the safety and efficacy of 100mg and 200mg filgotinib versus placebo on the basis of clinical remission and endoscopic response in a 10-week induction phase followed by a 47-week maintenance phase. Topline results from the study are expected in the first half of 2023.
We remind investors that Galapagos has a global collaboration agreement to develop and commercialize filgotinib with Gilead Sciences GILD.
Shares of the company have plunged 49.2% so far this year compared with the industry’s decrease of 10.9%.
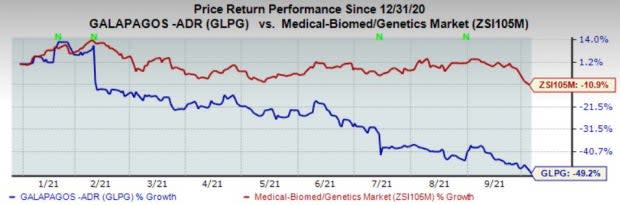
Image Source: Zacks Investment Research
Concurrently, Galapagos announced that it will assume sponsorship of and operational and financial responsibility for the ongoing DIVERSITY study, evaluating filgotinib for CD and its long-term extension study, in agreement with partner Gilead. Both parties expect to complete the transfer by Jun 30, 2022.
Per the terms of the deal, Gilead will make a one-time payment of $15 million to Galapagos in consideration for the latter assuming responsibility of the DIVERSITY clinical study. Galapagos will also be solely responsible for all the development costs of the DIVERSITY study effective Apr 1, 2022.
In addition, if the European Medicines Agency grants a regulatory approval for filgotinib to treat CD, based on data from the DIVERSITY trial, then royalties payable by Galapagos to Gilead will be reduced 30% across all filgotinib indications and will accrue in the range of 5.6-10.5% of net sales in Europe. These royalties are payable as of 2024. Gilead remains responsible for commercial activities outside Europe.
Filgotinib is marketed as Jyseleca in the European Union, Great Britain and Japan for the treatment of adults with moderate-to-severely active rheumatoid arthritis (RA) and inadequate response or intolerance to one or more disease-modifying anti-rheumatic drugs (DMARDs).
Applications were filed in the European Union, Great Britain and Japan for the label expansion of filgotinib to address the indication of ulcerative colitis (UC).
Earlier, Gilead announced its decision to not seek an approval for filgotinib to treat RA in the United States as it does not see a viable path for the drug regarding the same indication.
Separately, Galapagos announced results of two post-hoc analyses from the SELECTION and SELECTION LTE studies, which are part of its investigational clinical program for filgotinib to manage patients with moderately-to-severely active UC. Data showed clinical benefits of continued dosing with filgotinib 200mg in patients who did not respond at week 10.
The successful development of filgotinib for these inflammatory conditions will lend a significant boost to Galapagos.
Key drugs approved for CD include AbbVie’s ABBV Humira, which is also evaluating Skyrizi for the same. Eli Lilly and Company LLY is also evaluating mirikizumab for CD.
Galapagos currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Eli Lilly and Company (LLY) : Free Stock Analysis Report
Gilead Sciences, Inc. (GILD) : Free Stock Analysis Report
AbbVie Inc. (ABBV) : Free Stock Analysis Report
Galapagos NV (GLPG) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 