Biogen (BIIB) Partner Begins Filing for Leqembi SC Autoinjector
Biogen Inc.’s BIIB Japan-based partner, Eisai., announced that it has initiated the rolling submission of a biologics license application (“BLA”) to the FDA seeking approval for Leqembi (lecanemab) subcutaneous (SC) autoinjector as a weekly maintenance dosing for the treatment of early Alzheimer’s disease (AD).
Leqembi gained full approval from the FDA for early Alzheimer’s disease in the United States and broad reimbursement from the Centers for Medicare & Medicaid Services in July 2023. Leqembi is also approved in China and Japan.
Eisai initiated the rolling submission of the BLA after the Leqembi SC autoinjector version for weekly maintenance dosing was granted Fast Track designation by the regulatory body. The BLA was based on data from the Clarity AD open-label extension study and modeling of observed data.
Eisai had initially planned to file a supplemental biologics license application (sBLA) to the FDA for the SC formulation in March. However, the FDA then requested additional three-month immunogenicity data at the proposed maintenance dose of Leqembi 360 mg weekly. As a result, Eisai decided to initiate a rolling BLA for Leqembi SC maintenance under the existing Fast Track and Breakthrough Therapy designations.
However, the FDA guided Eisai to get a Fast Track designation specific to the SC formulation of Leqembi to receive a rolling review. Following the guidance from the FDA, Eisai submitted a request for Fast Track designation for the SC formulation.
In March, Eisai completed the submission of an sBLA to the FDA seeking approval for a maintenance intravenous (IV) dosing version of Leqembi.
Shares of Biogen have declined 12.3% year to date compared with the industry’s decrease of 5.3%.
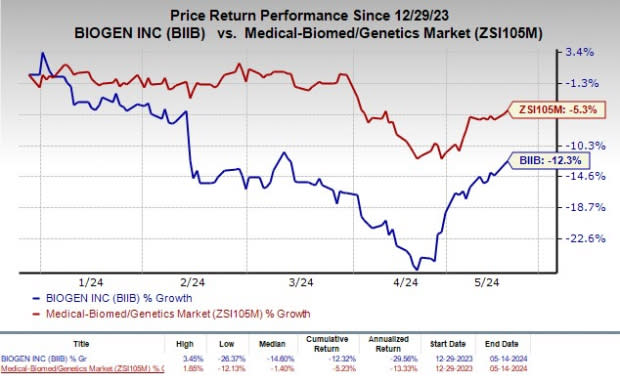
Image Source: Zacks Investment Research
Biogen has developed Leqembi in collaboration with Eisai, with the latter leading the clinical development and regulatory submissions. Though the companies co-commercialize and co-promote the drug, Eisai has the final decision-making authority.
If approved, the autoinjector SC version of Leqembi could be used by patients at home or at medical centers as the process takes less time than the IV formulation of the drug.
Meanwhile, in March 2024, the European Medicines Agency’s Committee for Medicinal Products for Human Use delayed the decision to approve Leqembi.
Though the Leqembi launch is progressing slowly, Biogen believes it has the potential to generate blockbuster sales. Biogen expects sales of Leqembi to start growing from 2024 with signs of acceleration seen in the first quarter. Eisai recorded nearly $19 million in sales from Leqembi in the first quarter of 2024, compared with $7 million recorded in the fourth quarter of 2023. The number of patients on Leqembi has also increased nearly 2.5 times since 2023-end.
Biogen believes that Leqembi has the potential to generate blockbuster sales as there is a huge unmet medical need for AD.
The AD target market is highly competitive as a few other pharma companies like Eli Lilly LLY and Prothena are developing their antibody candidates targeting the AD indication. The Alzheimer’s drugs of these companies are either under regulatory review or being evaluated in clinical studies.
Eli Lilly developed donanemab, its antibody therapy for AD, which is currently under review by the FDA. In March 2024, the FDA delayed its decision on the donanemab filing, which was initially expected before the end of first-quarter 2024.
Earlier this month, LLY announced that the regulatory body now intends to convene an in-person meeting of the Peripheral and Central Nervous System Drugs Advisory Committee on Jun 10, 2024, to discuss data from the phase III TRAILBLAZER-ALZ 2 study, which supports Lilly’s regulatory filing for donanemab.
Zacks Rank & Stock to Consider
Biogen currently has a Zacks Rank #3 (Hold).
A top-ranked stock in the healthcare sector is Third Harmonic Bio, Inc. THRD, sporting a Zacks Rank #1 (Strong Buy) at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
In the past 60 days, estimates for Third Harmonic Bio’s 2024 loss per share have narrowed from $1.65 to $1.35. Year to date, shares of THRD have rallied 17.7%.
THRD’s earnings beat estimates in three of the trailing four quarters and missed the same once, the average surprise being 37.72%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Biogen Inc. (BIIB) : Free Stock Analysis Report
Eli Lilly and Company (LLY) : Free Stock Analysis Report
Third Harmonic Bio, Inc. (THRD) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 