AstraZeneca's (AZN) Asthma Drug Tezepelumab Gets Nod in Europe
AstraZeneca AZN announced that the European Commission has granted approval to its monoclonal antibody, Tezspire (tezepelumab) for the treatment of severe asthma.
Tezspire can now be prescribed in Europe as an add-on maintenance treatment for patients aged 12 years and older with severe asthma who are inadequately controlled with high-dose inhaled corticosteroids plus another medicinal product. Tezspire was approved in the United States for a similar indication in December 2021. AstraZeneca markets Tezspire in partnership with Amgen AMGN.
The approval in Europe was expected as in July, The Committee for Medicinal Products for Human Use of the European Medicines Agency had given a positive opinion, recommending the marketing authorization of Tezspire.
So far this year, AstraZeneca’s shares have declined 3.1% compared with a decrease of 5.1% for the industry.
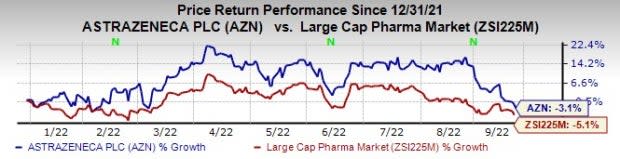
Image Source: Zacks Investment Research
The approval of Tezspire was based on data from the PATHFINDER clinical program and included the pivotal phase III NAVIGATOR study. Data from these studies showed that treatment with Tezspire led to superior results across every primary and key secondary endpoint in patients with severe asthma, compared to placebo, when added to standard therapy.
Patients with severe asthma experience frequent exacerbations and an increased risk of hospitalization. In the studies, which included a broad population of severe asthma patients, Tezspire consistently and significantly reduced exacerbations. It is the only biologic medicine approved by the FDA as well as EC to treat severe asthma with no phenotype limitation and irrespective of biomarker levels.
AstraZeneca and Amgen are jointly developing Tezspire. While AstraZeneca leads the development of the drug, Amgen leads manufacturing. Amgen and AstraZeneca will jointly commercialize Tezspire in North America. While Amgen will record product sales in the United States, AstraZeneca will record its share of profits in the United States as collaboration revenues. AstraZeneca will record product sales outside the United States while Amgen will record profit share.
AstraZeneca and Amgen are also evaluating Tezspire for other indications like chronic obstructive pulmonary disease (COPD), chronic rhinosinusitis with nasal polyps, chronic spontaneous urticaria and eosinophilic esophagitis (EoE).
Competitive pressure in the overall asthma market has intensified over the years. Tezspire is likely to face stiff competition from Glaxo’s GSK Nucala, and Regeneron REGN and Sanofi’s blockbuster medicine, Dupixent, which are approved for treating patients with severe asthma and some other indications as well.
Other than severe eosinophilic asthma, Glaxo’s Nucala is approved for hypereosinophilic syndrome, eosinophilic granulomatosis and polyangiitis and chronic rhinosinusitis with nasal polyps.
Nucala is a key top-line driver for Glaxo. It is being studied in late-stage studies for COPD.
Sanofi and Regeneron’s Dupixent is now approved in the United States and the EU for three type II inflammatory diseases, namely severe chronic rhinosinusitis with nasal polyposis, severe asthma and moderate-to-severe atopic dermatitis. It was recently approved for the fourth indication, EoE in the United States.
With outside U.S. revenues accelerating and multiple approvals for new indications expected in the near future, Dupixent’s sales are expected to be higher. Sanofi and Regeneron’s Dupixent is in late-stage development for bullous pemphigoid, chronic spontaneous urticaria, and COPD. It is also under priority review in the United States for prurigo nodularis.
AstraZeneca currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Regeneron Pharmaceuticals, Inc. (REGN) : Free Stock Analysis Report
AstraZeneca PLC (AZN) : Free Stock Analysis Report
GSK PLC Sponsored ADR (GSK) : Free Stock Analysis Report
Amgen Inc. (AMGN) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 