Annovis (ANVS) Gains on FDA Authorization for AD Study
Shares of Annovis Bio, Inc. ANVS gained 2.39% on Oct 6 after the FDA authorized the company to proceed with the phase II/III clinical study of lead candidate buntanetap in moderate Alzheimer's Disease (AD).
Buntanetap (previously known as ANVS401 or Posiphen) is an oral translational inhibitor of neurotoxic aggregating proteins (TINAPs). Buntanetap inhibited the synthesis of neurotoxic proteins — amyloid precursor protein APP/Aβ (APP), tau/phospho-tau (tau) and αSynuclein (αSYN) — that are the main causes of neurodegeneration.
Annovis requested approval from the FDA to further pursue the development of buntanetap in AD following the submission of the phase IIa safety data and the chronic toxicology data in animals.
Consequently, the FDA approved the company's development plan and study protocol, and authorized the initiation of phase II/III study of buntanetap in AD.
The candidate was shown to be well-tolerated and safe, and its pharmacokinetics were found to be in line with levels measured earlier in humans, meeting both the primary and secondary endpoints in a phase IIa study in AD and Parkinson's disease (PD) patients. Buntanetap resulted in statistically significant improvement in motor function in PD patients and cognition in AD patients. Thereafter, the company advanced buntanetap into a phase III trial for the treatment of early PD.
Annovis shares have lost 26.3% in the year-to-date period compared with the industry’s 25.8% decline.
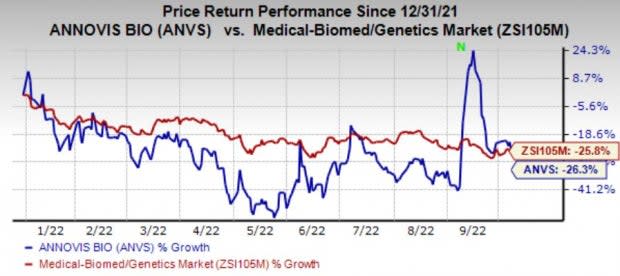
Image Source: Zacks Investment Research
In August 2022, Annovis dosed the first patient in the phase III study evaluating buntanetap in early PD.
The AD space is in the spotlight as Biogen BIIB and partner Eisai recently announced that their big global phase III confirmatory study, Clarity AD, on investigational anti-amyloid beta protofibril antibody candidate lecanemab (BAN2401) for treating early AD, met the primary endpoint. The study's success is a landmark and has boosted investor sentiment.
AD is a progressive, fatal disease of the brain characterized by a decline in memory, language and other thinking skills, as well as changes in mood and behavior.
We remind investors that the FDA’s approval of Biogen’s and Eisai’s controversial AD drug, Aduhelm, in June 2021 put the spotlight on companies having promising AD candidates in their pipeline last year as well. The euphoria regarding Aduhelm, however, died down as the drug witnessed a slow launch due to reimbursement issues as its efficacy lwas under the scanner.
Nevertheless, despite the complexities associated with developing a treatment for AD and past failures, the space will continue to attract attention from pharma and biotech companies, given the significant unmet need.
Pharma giant Eli Lilly LLY and clinical-stage biotech Prothena PRTA also have promising AD candidates in their pipeline.
Lilly is evaluating donanemab, an investigational antibody that targets a modified form of beta-amyloid called N3pG for treating AD. The candidate is under priority review in the United States for AD under the accelerated approval pathway. Given the success of Biogen/Eisai’s AD candidate, the chances of Lilly’s AD drug approval have become high.
Prothena is evaluating PRX012, an investigational high-potency monoclonal antibody targeting a key epitope at the N-terminus of amyloid beta (Aβ) for treating AD. Prothena is also evaluating PRX005 in collaboration with Bristol Myers. PRX005 — a potential treatment for AD — is an investigational antibody that targets tau, a protein implicated in diseases like AD, frontotemporal dementia, progressive supranuclear palsy, chronic traumatic encephalopathy and other tauopathies.
The company is also developing a dual Aβ-Tau vaccine — a potential prevention and treatment for AD — to target key epitopes within Aβ and tau proteins to promote amyloid clearance and block pathogenic tau interaction. An investigational new drug (IND) application for the vaccine is anticipated in 2023.
Annovis has a Zacks Rank #3 (Hold) currently. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Biogen Inc. (BIIB) : Free Stock Analysis Report
Eli Lilly and Company (LLY) : Free Stock Analysis Report
Prothena Corporation plc (PRTA) : Free Stock Analysis Report
Annovis Bio, Inc. (ANVS) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 