Abbott (ABT) Gets FDA Nod for Two Glucose Monitoring Systems
Abbott ABT announced that Lingo and Libre Rio, two of its glucose monitoring system, have received FDA clearance. Lingo and Libre Rio are based on Abbott’s own Freestyle Libre continuous glucose monitoring (CGM) technology.
The latest development is expected to expand the company’s Diabetes Care business.
More in the News
Both the consumer biowearable devices are designed to cater to different needs. Lingo is for consumers who are 18 years or older and want a better understanding about their metabolic health, including personalized coaching to promote lifestyle changes. Libre Rio is for consumption by adults aged 18 or above with Type 2 diabetes, who do not use insulin and maintain their health through lifestyle alterations.
About Lingo
Lingo is designed for the population intending to improve health and wellness. The device consists of biosensor and needs to be worn on upper arm for 14 days. It will then track glucose levels from the body’s reaction to different foods consumed, exercise and daily stressors. The device is linked with a coaching application on a smartphone, which will provide necessary customized advice for consumers’ better health and well-being.
In this regard, Abbott noted that as per a study by the University of North Carolina, just 12% of Americans are metabolically healthy based on five key indicators of metabolic health, including glucose levels, suggesting that most of the U.S. population has room to improve their metabolism. This reflects the huge potential for Lingo.
Further, the device is expected to witness huge market acceptance. This is because, according to a recent online consumer survey by The Harris Poll (on behalf of Abbott), 82% of Americans are eager to change their lifestyle with the help of a biowearable like Lingo.
About Libre Rio
Libre Rio will be part of Abbott’s libre portfolio of CGM system available in the United States. Designed for adults with Type 2 diabetes, this device is aimed at improving their health condition through lifestyle modification and not with the use of insulin. It comes with a measurement range of 40-400mg/dL, capturing extremely low or high glucose level. Unlike other Freestyle Libre devices, Libre Rio does not require any prescription, which makes it more user-friendly.
According to an American Diabetes Association report, diabetes is among the top public health challenges in the United States, with nearly 38.4 million people living with the condition.
Abbott’s FreeStyle Libre systems are already available over-the-counter in more than 50 countries. However, in the United States, they are only available through prescriptions, creating limited access for patients. Libre Rio, as a non-prescription biowearable, is expected to gain huge market acceptance.
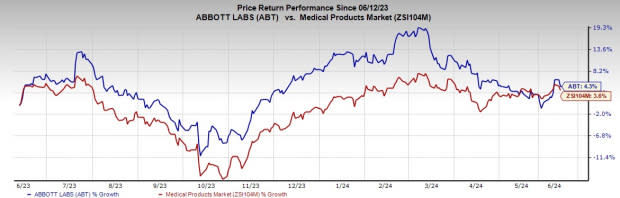
Image Source: Zacks Investment Research
Industry Prospects
As per a Future Market Insights report, the global CGM systems market was worth $ 11.1 billion in 2023, and is expected to reach $103.5 billion by 2033, at a CARG of 25.1%. Primary factors influencing this huge market prospect include the rising prevalence of diabetes globally, technological advancement, and increasing adoption of CGM systems because of their user-friendly, reliable and accurate approach to measuring glucose level.
Other Recent Development
In June 2024, AVEIR DR, the world’s first dual chamber leadless pacemaker system, received a CE Mark in Europe. AVEIR DR is a notable Abbott product that treats people with abnormal or slow heart rhythms. This technology redefines Abbott’s process of treating abnormal heart rhythms.
In April 2024, the FDA approved the Esprit BTK Everolimus Eluting Resorbable Scaffold System, making it a significant recognition of Abbott’s effort toward peripheral artery disease below the knee. There were no stents or drug-coated balloons approved for use below the knee in the United States until that day.
Price Performance
Over the past year, ABT stock has risen 4.3%, compared to the industry’s 3.6% growth.
Zacks Rank and Key Picks
ABT currently carries a Zacks Rank #3 (Hold).
Some better-ranked stocks in the broader medical space are Hims & Hers Health HIMS, Medpace MEDP and ResMed RMD. While Hims & Hers Health and Medpace sport a Zacks Rank #1 (Strong Buy) each, ResMed carries a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks Rank #1 stocks here.
The Hims & Hers Heath stock has surged 143.8% in the past year. Estimates for the company’s earnings have remained constant at 18 cents for 2024 and increased 3.1% to 33 cents for 2025 in the past 30 days.
HIMS’ earnings beat estimates in three of the trailing four quarters and missed in one, delivering an average surprise of 79.2%. In the last reported quarter, it posted an earnings surprise of a staggering 150%.
Estimates for Medpace’s 2024 earnings per share have moved up to $11.29 from $11.23 in the past 30 days. Shares of the company have surged 81.4% in the past year compared with the industry’s 4.2% growth.
MEDP’s earnings surpassed estimates in each of the trailing four quarters, the average surprise being 12.8%. In the last reported quarter, it delivered an earnings surprise of 30.6%.
Estimates for ResMed’s fiscal 2024 earnings per share have remained constant at $7.70 in the past 30 days. Shares of the company have lost 2.1% in the past year against the industry’s growth of 3.9%.
RMD’s earnings surpassed estimates in three of the trailing four quarters and missed in one, the average surprise being 2.8%. In the last reported quarter, it delivered an earnings surprise of 10.9%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Abbott Laboratories (ABT) : Free Stock Analysis Report
ResMed Inc. (RMD) : Free Stock Analysis Report
Medpace Holdings, Inc. (MEDP) : Free Stock Analysis Report
Hims & Hers Health, Inc. (HIMS) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 