Ultragenyx's (RARE) Q2 Earnings Miss Estimates, Revenues Up
Ultragenyx Pharmaceutical Inc. RARE incurred a loss of $2.26 per share in second-quarter 2022, wider than the Zacks Consensus Estimate of a loss of $1.72. The company reported a loss of $1.45 per share in the year-ago quarter.
Ultragenyx’s total revenues of $89.34 million in the second quarter, up 2.7% year over year. The top line surpassed the Zacks Consensus Estimate of $89 million.
Shares of Ultragenyx have lost 38.3% in the year so far compared with the industry's decline of 18.1%.
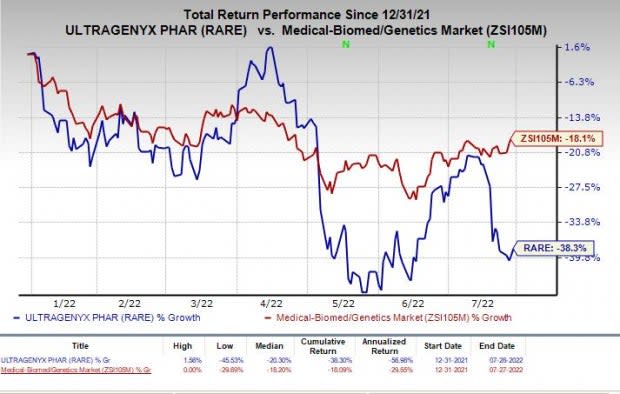
Image Source: Zacks Investment Research
Ultragenyx markets three drugs, namely Crysvita, Mepsevii and Dojolvi. Crysvita is approved for treating X-linked hypophosphatemia, an inherited disorder; and tumor-induced osteomalacia, an ultra-rare disease. Mepsevii is approved to treat Mucopolysaccharidosis VII (MPS VII), also known as Sly syndrome. Dojolvi was approved in June 2020 for all forms of long-chain fatty acid oxidation disorders (LC-FAOD).
Quarter in Detail
Crysvita’s total revenues were $69.4 million, up 40.1% year over year, driven by increased demand for approved indications. Crysvita revenues in Ultragenyx territories rose 43.3% to $64 million in the second quarter and included $51.6 million from the North America profit share territory and $12.4 million of net product sales for the drug in other regions. Total royalty revenues related to the sales of Crysvita in the European region were $5.4 million. Ultragenyx sold its Crysvita rights in the European territory to Royalty Pharma in December 2019.
Mepsevii product revenues were $4.9 million in the second quarter compared with $5.4 million reported in the year-ago quarter. Dojolvi product revenues were $13.5 million compared with $10 million in the year-ago quarter, driven by strong new patient demand.
Operating expenses increased 36% to $230.9 million in the second quarter due to pipeline advancements.
2022 Guidance
Ultragenyx reaffirmed the guidance it had provided earlier this year. The company expectsCrysvita revenues in the range of $250-$260 million in Ultragenyx territories. Dojolvi revenues are expected to be $55-$65 million
Pipeline Updates
In May, Ultragenyx announced positive long-term safety and efficacy data from the phase I/II studies of its candidates DTC401 and DTC301 for the potential treatment of Glycogen Storage Disease Type Ia (GSDIa) and the potential treatment of Ornithine Transcarbamylase (OTC) deficiency, respectively. Results to date exhibited ongoing durability of both safety and response for both the gene therapy programs.
In April, Ultragenyx dosed the first patient in a pivotal phase II/III study evaluating its fully human monoclonal antibody, setrusumab (UX143), for the treatment of osteogenesis imperfecta (“OI”) in patients aged between five to less than 26 years.
In the second half of 2022, the company expects to provide a dosing update on the phase II portion of the Orbit study and transition to phase III.
Other Updates
Ultragenyx recently sold 30% of the royalty interests it receives from partner Kyowa Kirin on future sales of its rare disease drug, Crysvita, in the United States and Canada. In return, RARE will receive $500 million without diluting capital. This money will be used by Ultragenyx to fund its ongoing clinical studies as well as planned commercial activities.
In May, Ultragenyx entered into an exclusive license agreement with New York-based biopharmaceutical company, Abeona Therapeutics Inc. ABEO, wherein it will acquire global rights of the latter’s gene therapy candidate ABO-102 (now UX111). The AAV gene therapy candidate, UX111, is currently being evaluated in the open-label, global phase I/II Transpher A study for the treatment of patients with Sanfilippo syndrome type A (MPS IIIA), a rare, fatal lysosomal storage disease.
Ultragenyx is evaluating UX701, an AAV type-9 gene therapy product candidate, in a seamless phase I/II/III study for the treatment of Wilson disease — a larger rare metabolic disease.
Ultragenyx Pharmaceutical Inc. Price, Consensus and EPS Surprise
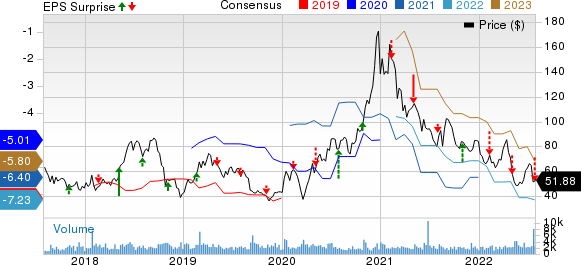
Ultragenyx Pharmaceutical Inc. price-consensus-eps-surprise-chart | Ultragenyx Pharmaceutical Inc. Quote
Zacks Rank & Stocks to Consider
Ultragenyx currently carries a Zacks Rank #3 (Hold).
Some better-ranked stocks in the biotech sector is BioNTech BNTX and Scholar Rock SRRK, each carrying a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
BioNTech’s earnings estimates for 2022 have declined from $34.82 to $33.51 in the past 30 days. Shares of BioNTech have declined 35.4% year to date. Earnings of BNTX beat earnings estimates in all the last four quarters, witnessing a surprise of 56.87%, on average.
Scholar Rock’s loss estimates for 2022 have narrowed from $2.59 to $2.48 in the past 30 days. Shares of SRRK have declined 70.1% year to date. Earnings of BNTX beat earnings estimates in two of the trailing four quarters and missed the same in the remaining two occasions, witnessing a surprise of 13.12%, on average.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Ultragenyx Pharmaceutical Inc. (RARE) : Free Stock Analysis Report
Abeona Therapeutics Inc. (ABEO) : Free Stock Analysis Report
Scholar Rock Holding Corporation (SRRK) : Free Stock Analysis Report
BioNTech SE Sponsored ADR (BNTX) : Free Stock Analysis Report
To read this article on Zacks.com click here.

 Yahoo Finance
Yahoo Finance 