Ultragenyx's (RARE) GTX-102 Gets FDA Nod for Uniform Dosing
Ultragenyx Pharmaceutical Inc. RARE announced that the FDA has agreed to a protocol amendment, after review, of the phase I/II study of GTX-102 in pediatric patients with Angelman syndrome, a rare neurogenetic disorder. This ruling by the regulatory body will enable Ultragenyx to harmonize dose ranges in the United States with those being used in study cohorts outside the United States.
The company has reportedly been actively enrolling and dosing patients in the expansion cohorts to verify the GTX-102 dose and treatment regimen to be used in the subsequent phase III program.
Ultragenyx’s stock climbed 5.3% on Wednesday, following the positive news. In the past year, shares of Ultragenyx have gained 4.5% against the industry’s 5.5% decline.
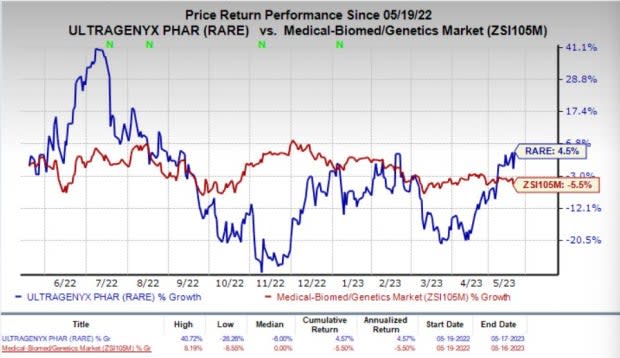
Image Source: Zacks Investment Research
Ultragenyx is developing GTX-102 for treating patients with Angelman syndrome. GTX-102 has been granted Orphan Drug Designation, Rare Pediatric Disease Designation and Fast Track Designation by the FDA.
The objective of the phase I/II dose-escalating study is to evaluate the safety and tolerability profile of GTX-102 in pediatric patients with Angelman syndrome with a genetically confirmed diagnosis of full maternal UBE3A gene deletion. Patients, who have been dosed early in the study, have now moved into a long-term maintenance dosing. As of May 2023, 13 patients have had more than 12 months of exposure to GTX-102, with the longest being more than 18 months.
Management claims that the FDA’s nod regarding the protocol amendment will speed up the study process as comparable dose ranges will now be administered across all geographies. The company has been working on activating study sites in the United States to begin enrollment as soon as possible.
Outside the United States, Ultragenyx is currently engaged in enrolling patients in expansion cohorts of the study. The company plans to enroll approximately 40 patients across all expansion cohorts in and outside the United States.
Ultragenyx Pharmaceutical Inc. Price and Consensus

Ultragenyx Pharmaceutical Inc. price-consensus-chart | Ultragenyx Pharmaceutical Inc. Quote
Zacks Rank and Stocks to Consider
Ultragenyx currently carries a Zacks Rank #3 (Hold).
Some better-ranked stocks in the biotech sector are Allogene Therapeutics ALLO, Anixa Biosciences ANIX and ADMA Biologics, Inc. ADMA, each carrying a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
In the past 90 days, the Zacks Consensus Estimate for Allogene Therapeutics’ 2023 loss per share has narrowed from $2.83 to $2.32. In the past year, shares of Allogene Therapeutics have fallen by 18.9%.
ALLO beat estimates in three of the trailing four quarters, missing the mark on one occasion, delivering an average earnings surprise of 5.08%.
In the past 90 days, the Zacks Consensus Estimate for Anixa Therapeutics’ 2023 loss per share has narrowed from 62 cents to 43 cents. In the past year, shares of Anixa Therapeutics have increased by 2.4%.
ANIX beat estimates in each of the trailing four quarters, delivering an average earnings surprise of 24.04%.
In the past 90 days, the Zacks Consensus Estimate for ADMA Biologics’ 2023 loss per share has narrowed from 19 cents to 9 cents. In the past year, shares of ADMA Biologics have increased by 100.5%.
ADMA beat estimates in each of the trailing four quarters, delivering an average earnings surprise of 19.13%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Ultragenyx Pharmaceutical Inc. (RARE) : Free Stock Analysis Report
ADMA Biologics Inc (ADMA) : Free Stock Analysis Report
ANIXA BIOSCIENCES INC (ANIX) : Free Stock Analysis Report
Allogene Therapeutics, Inc. (ALLO) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 