Sarepta (SRPT) Q1 Earnings Beat Estimates, Revenues Up Y/Y
Sarepta Therapeutics, Inc. SRPT reported an adjusted loss of 97 cents per share in the first quarter of 2023, narrower than the Zacks Consensus Estimate and our estimate of a loss of $1.46 and $1.48, respectively. However, the figure was wider than the year-ago quarter’s loss of 56 cents per share.
Sarepta recorded total revenues of $253.5 million, up 20.2% year over year. Revenues beat the Zacks Consensus Estimate and our model estimate of $223.2 million and $221.4 million, respectively. The year-over-year increase in revenues was driven by the sales of Sarepta’s three currently-approved RNA-based PMO therapies for Duchenne muscular dystrophy (DMD).
In the year so far, Sarepta’s shares have declined 4.6% compared with the industry’s 5.4% fall.
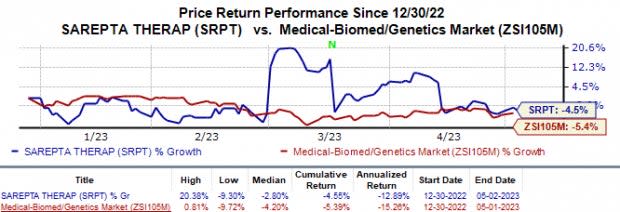
Image Source: Zacks Investment Research
Quarter in Detail
Sarepta’s commercial portfolio includes three drugs approved for treating Duchenne muscular dystrophy (“DMD”) — Exondys 51, Vyondys 53 and Amondys 45.
The company derived product revenues of $231.5 million, up 23.0% year over year. The upside was driven by an increase in demand for its DMD products.
The company recorded $22.0 million in collaboration revenues, primarily from its licensing agreement with Roche RHHBY. In the year-ago period, management had recorded $22.0 million as collaboration revenues, which were also received from Roche.
Sarepta and Roche entered into a licensing agreement to develop SRP-9001, its investigational gene-therapy candidate for DMD, in 2019. Per the agreement, Roche has exclusive rights to launch and commercialize SRP-9001 in ex-U.S. markets.
Adjusted research and development (R&D) expenses totaled $220.7 million in the first quarter, up 27.5% year over year. This surge is attributable to the increase in manufacturing expenses for ramping up production for SRP-9001.
Adjusted selling, general & administrative (SG&A) expenses were $83.3 million, up 56.6% year over year. The upside was driven primarily by an increase in professional service expenses incurred by the company to prepare for a potential launch of SRP-9001.
2023 Guidance
The company reiterates its product revenue guidance for the full year. Management expects to generate more than $925 million in product revenues from its three approved PMO therapies.
Pipeline Updates
Last November, Sarepta and partner Roche announced that the FDA accepted the biologic license application (BLA) seeking accelerated approval for SRP-9001 in DMD. The BLA has been granted priority review and a final decision is expected before the end of this month. Prior to the final decision, the agency scheduled a meeting of its Cellular, Tissue and Gene Therapies Advisory Committee on May 12, 2023, to discuss the SRP-9001 BLA.
Alongside Q1 earnings, Sarepta also announced that it has completed the enrolment in part B of the pivotal phase II MOMENTUM study evaluating its next-generation PPMO-technology, SRP-5051, in DMD patients amenable to exon 51 skipping. Data from this study is expected in second-half 2023. If the study is successful, Sarepta anticipates using data from this study to seek accelerated approval for the candidate.
In February, management started the phase I VOYAGENE study, which is evaluating its gene therapy candidate, SRP-9003, for the treatment of limb-girdle muscular dystrophy Type 2E (LGMD2E). The study will enroll ambulant and non-ambulant patients. The company expects to complete enrolment in the study in second-half 2023 and start a late-stage study on the candidate before this year’s end.
Sarepta Therapeutics, Inc. Price
Sarepta Therapeutics, Inc. price | Sarepta Therapeutics, Inc. Quote
Zacks Rank & Stocks to Consider
Sarepta currently has a Zacks Rank #3 (Hold). A couple of better-ranked stocks in the overall healthcare sector include Allogene ALLO and Ligand Pharmaceuticals LGND, each carrying a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Allogene’s stock has declined 14.9% in the year-to-date period. Allogene’s loss estimates for 2023 have narrowed from $2.56 to $2.44 per share in the past 60 days. During the same period, the loss estimates per share for 2024 have improved from $2.53 to $2.46.
Allogene beat earnings estimates in each of the last four quarters, the average surprise being 8.33%. In the last reported quarter, the company delivered an earnings surprise of 7.04%.
In the past 60 days, estimates for Ligand Pharmaceuticals’ 2023 EPS have increased from $3.61 to $4.16. During the same period, the earnings estimates per share for 2024 have risen from $3.58 to $4.58. Shares of Ligand Pharmaceuticals have gained 11.8% in the year-to-date period.
Earnings of Ligand beat estimates in one of the last four quarters, while missing the mark on the other three occasions. On average, the company’s earnings witnessed a negative surprise of 10.07%. In the last reported quarter, Ligand’s earnings beat estimates by 10.57%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Roche Holding AG (RHHBY) : Free Stock Analysis Report
Ligand Pharmaceuticals Incorporated (LGND) : Free Stock Analysis Report
Sarepta Therapeutics, Inc. (SRPT) : Free Stock Analysis Report
Allogene Therapeutics, Inc. (ALLO) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 