Roche (RHHBY) Tecentriq Meets Adjuvant Liver Cancer Study Goal
Roche Holding AG RHHBY announced data from the pivotal phase III IMbrave050 study evaluating its cancer drug, Tecentriq, in combination with Avastin as adjuvant treatment following surgery for people with early-stage hepatocellular carcinoma (HCC) who are at high risk of disease recurrence. HCC is the most common type of liver cancer.
Data from the study showed that treatment with Tecentriq plus Avastin led to a statistically significant improvement in recurrence-free survival (RFS) in HCC patients who have an increased risk of recurrence following resection or ablation with curative intent compared with active surveillance. The study met its primary endpoint of RFS at the prespecified interim analysis.
Per the company, overall survival data were immature at the time of interim analysis and the follow-up will continue till the time of the next analysis.
The safety profile for Tecentriq and Avastin was similar to the safety profile of each therapeutic agent and with the underlying disease.
RHHBY plans to discuss the above-mentioned data with the FDA and the European Medicines Agency, as well as other health authorities worldwide. The company plans to present data from the IMbrave050 study at an upcoming medical conference.
Shares of Roche have declined 19.6% in the past year against the industry’s rally of 15.1%.
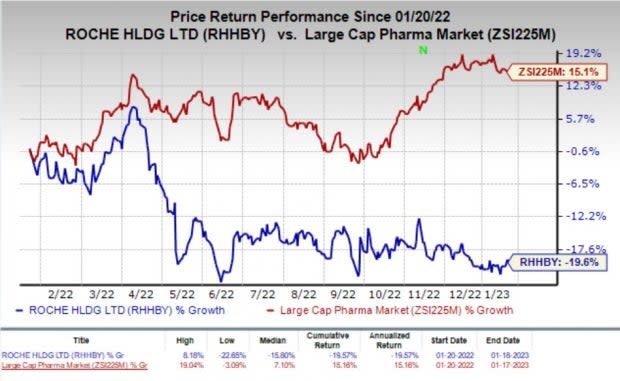
Image Source: Zacks Investment Research
Roche’s Tecentriq is a leading immune-oncology drug approved for multiple indications.
Tecentriq is approved in the European Union and the United States for previously-treated metastatic NSCLC and certain types of untreated or previously-treated metastatic urothelial carcinoma (mUC), HCC and other cancer indications. The FDA had approved the drug in combination with Avastin and chemotherapy for the initial treatment of people with metastatic non-squamous NSCLC. Tecentriq is approved for the first-line treatment of extensive-stage small cell lung cancer (ES-SCLC).
Roche has an extensive development program for Tecentriq, including multiple ongoing and planned phase III studies across lung, genitourinary, skin, breast, gastrointestinal, gynecological, and head and neck cancers. The program includes studies evaluating Tecentriq alone and in combination with other drugs. Label expansion of the drug is likely to boost sales.
However, Tecentriq faces stiff competition from Merck’s MRK blockbuster cancer drug, Keytruda, and Bristol Myers’ BMY Opdivo, which are approved for similar indications.
Merck’s biggest revenue generator, Keytruda, is approved for treating several types of cancer indications. MRK continues to study Keytruda for addressing more cancer indications.
Opdivo is one of the top revenue generators for Bristol Myers, which is approved for treating several types of cancer indications. BMY continues to study Opdivo for addressing more cancer indications.
Zacks Rank & Another Stock to Consider
Roche currently carries a Zacks Rank #2 (Buy). Another top-ranked stock in the biotech sector is Syndax Pharmaceuticals, Inc. SNDX, sporting a Zacks Rank #1 (Strong Buy) at present.You can see the complete list of today’s Zacks #1 Rank stocks here.
Loss per share estimates for Syndax Pharmaceuticals have narrowed 6.5% for 2023 in the past 60 days.
Earnings of Syndax Pharmaceuticals surpassed estimates in three of the trailing four quarters and met the same on the other occasion. SNDX witnessed an earnings surprise of 95.39%, on average.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Roche Holding AG (RHHBY) : Free Stock Analysis Report
Bristol Myers Squibb Company (BMY) : Free Stock Analysis Report
Merck & Co., Inc. (MRK) : Free Stock Analysis Report
Syndax Pharmaceuticals, Inc. (SNDX) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 