Roche (RHHBY) Polivy Combination for DLBCL Gets Full Approval
Roche RHHBY announced that the FDA has approved Polivy (polatuzumab vedotin-piiq) in combination with Rituxan (rituximab), cyclophosphamide, doxorubicin and prednisone (R-CHP) for the treatment of adult patients who have previously untreated diffuse large B-cell lymphoma (DLBCL) not otherwise specified (NOS) or high-grade B-cell lymphoma (HGBL) and who have an International Prognostic Index (IPI) score of two or greater.
In 2019, the FDA granted accelerated approval to Polivy in combination with bendamustine plus Rituxan (BR) for the treatment of adults with relapsed or refractory (R/R) DLBCL who have received at least two prior therapies. The accelerated approval was granted for this indication based on complete response rates observed in a randomized, controlled clinical trial.
The latest FDA approval converts this accelerated approval to regular approval. Earlier, the FDA Oncologic Drugs Advisory Committee (ODAC) voted 11 to 2 in favor of Polivy in combination with R-CHP for previously untreated DLBCL.
The FDA approval of Polivy plus R-CHP for the first-line treatment of DLBCL is based on pivotal data from an international phase III, randomized, double-blind, placebo-controlled study, POLARIX, that demonstrated a statistically significant and clinically meaningful improvement in PFS compared with MabThera/Rituxan plus cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP). The risk of disease progression, relapse or death was reduced by 27% with Polivy plus R-CHP compared with R-CHOP.
Polivy in combination with R-CHP for previously untreated DLBCL is approved in quite a few countries.
Meanwhile, Roche continues to evaluate combinations of Polivy with the company’s CD20xCD3 T-cell engaging bispecific antibodies Lunsumio (mosunetuzumab) or Columvi (glofitamab). Trials include the phase III SUNMO study in combination with Lunsumio in patients with R/R DLBCL and the phase III POLARGO study with MabThera/Rituxan in combination with gemcitabine and oxaliplatin in patients with R/R DLBCL.
Roche’s hematology portfolio includes MabThera/Rituxan (rituximab), Gazyva/Gazyvaro (obinutuzumab), Polivy (polatuzumab vedotin-piiq), Venclexta/Venclyxto (venetoclax) in collaboration with AbbVie, Hemlibra (emicizumab), Lunsumio (mosunetuzumab) and Columvi (glofitamab).
Roche’s stock has lost 18% in the past year against the industry’s growth of 9%.
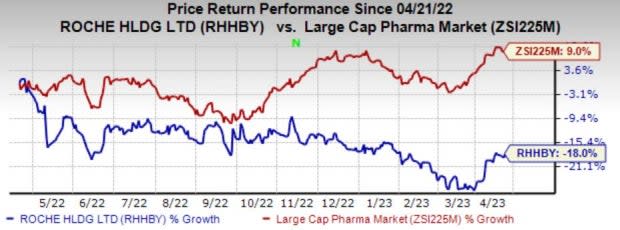
Image Source: Zacks Investment Research
Roche’s performance in 2022 was average, as the demand for COVID-19 products declined significantly, even though the diagnostics base business and newer drugs maintained their growth.
New drugs, namely Ocrevus, Hemlibra, Evrysdi and Tecentriq, recorded growth and the uptake of the new eye drug Vabysmo (launched at the beginning of 2022) was outstanding. This momentum should continue in 2023. The company is scheduled to provide a first-quarter sales update on Apr 26.
Roche currently carries a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Some other top-ranked stocks in the overall healthcare sector are Novo Nordisk NVO and Ligand Therapeutics LGND, both sporting a Zacks Rank #1 at present.
In the past 30 days, estimates for Novo Nordisk’s 2023 earnings per share have risen from $4.20 to $4.43 and estimates for 2024 have moved up by 29 cents to $5.19.
Ligand’s earnings per share estimates for 2023 increased to $4.32 from $3.30 in the past 30 days. LGND beat earnings estimates in one of the last four reported quarters and missed in the remaining three.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Roche Holding AG (RHHBY) : Free Stock Analysis Report
Novo Nordisk A/S (NVO) : Free Stock Analysis Report
Ligand Pharmaceuticals Incorporated (LGND) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 