Pharma Stock Roundup: FDA Okays PFE RSV Jab for Elderly, AZN Ends Brazikumab Studies
This week, the FDA approved Pfizer’s PFE RSV vaccine for older adults and AstraZeneca AZN and Merck’s MRK Lynparza for BRCA-mutated metastatic castration-resistant prostate cancer (mCRPC). The European Commission approved Novartis’ (NVS) IL-17A inhibitor, Cosentyx, for a new indication — hidradenitis suppurativa. J&J JNJ submitted a regulatory application to the FDA seeking approval for a single-tablet combination therapy of Opsumit for the long-term treatment of PAH. Sanofi’s SNY phase II study on a novel pipeline candidate, frexalimab, for treating relapsing multiple sclerosis met the primary endpoint.
Recap of the Week’s Most Important Stories
FDA Approves Pfizer’s RSV Vaccine for Older Adults: The FDA approved Pfizer’s RSV vaccine called Abrysvo (RSVpreF) for older adults. The vaccine is approved for the prevention of lower respiratory tract disease (LRTD) caused by RSV in individuals 60 years and older. The FDA approval for the RSV vaccine for older adults was based on data from the RENOIR study, which had enrolled approximately 37,000 participants. In February, the FDA’s Vaccines and Related Biological Products Advisory Committee (VRBPAC) voted in favor of RSVpreF for older adults. The committee voted 7 to 4 on safety and 7 to 4 on effectiveness.
Pfizer has also developed an RSV vaccine for infants through maternal immunization. The vaccine candidate is under FDA review, with a decision expected in August. Pfizer’s RSV vaccine candidate is also under review for both older adults and maternal immunization in Europe.
Pfizer’s pivotal phase III study evaluating its novel anti-tissue factor pathway inhibitor, marstacimab for treating hemophilia A and B patients without inhibitors to Factor VIII or Factor IX, met its primary endpoints. Data from the study demonstrated that prophylactic treatment with marstacimab led to a statistically significant and clinically relevant reduction in annualized bleeding rate (ABR) compared to prophylaxis and on-demand intravenous regimens in the abovementioned patient group. Study participants, who received factor replacement therapy in the six-month lead-in period, achieved a 92% reduction in bleeds upon switching to marstacimab. Data from the BASIS study also showed the superiority of marstacimab over the prophylaxis regimen, achieving a 35% reduction in ABR.
AstraZeneca Discontinues Studies on Brazikumab: AstraZeneca announced that it is discontinuing the Crohn’s disease (CD) and ulcerative colitis (UC) studies on its an anti-IL-23 monoclonal antibody, brazikumab. The candidate was being developed in a phase IIb/III INTREPID study in CD and the phase II EXPEDITION study in UC under the inflammatory bowel disease (IBD) development program. AstraZeneca has decided to discontinue the IBD development program on brazikumab as it believes the competitive landscape is evolving while the regulatory timeline of brazikumab has been delayed. AstraZeneca had acquired global rights to brazikumab from erstwhile Allergan, which the latter had divested in connection with its 2020 merger with AbbVie. AbbVie was funding the ongoing brazikumab study but it will now end.
The FDA approved AstraZeneca and partner Merck’s Lynparza for expanded use in first-line prostate cancer. The FDA approved Lynparza in combination with J&J’s Zytiga (abiraterone) and corticosteroid prednisone or prednisolone for patients with deleterious or suspected deleterious BRCA-mutated mCRPC, based on data from the PROpel study. In the PROpel study, the Lynparza combination led to improved radiographic progression-free survival and overall survival in the subgroup of patients with BRCA-mutated mCRPC.
In April, the FDA’s Oncologic Drugs Advisory Committee (ODAC) voted 11 to 1, with one abstention, in favor of approving Lynparza for the abovementioned expanded use in prostate cancer. The committee voted in favor of the FDA restricting the use of the Lynparza combination to these BRCA-mutated mCRPC patients, and not give approval for a broad range of patients with mCRPC. The FDA followed the advisory committee’s opinion.
Lynparza is already approved in the European Union and several other countries for the treatment of adult patients with mCRPC, based on the PROpel study. In the United States, Lynparza is presently approved for homologous recombination repair gene-mutated mCRPC patients who have progressed following prior treatment with enzalutamide or abiraterone based on the phase III PROfound study.
Sanofi’s Relapsing Multiple Sclerosis Study on Frexalimab Meets Goal: Sanofi announced data from a phase II study on its novel investigational anti-CD40L antibody, frexalimab (SAR441344) in relapsing multiple sclerosis at the annual meeting of the 2023 Consortium of Multiple Sclerosis Centers (CMSC). The data showed that frexalimab significantly reduced disease activity in patients with relapsing multiple sclerosis.
Following 12 weeks of treatment with a higher dose of frexalimab, patients experienced an 89% reduction in the number of new gadolinium-enhancing (GdE) T1-lesions, compared with placebo, thereby meeting the study’s primary endpoint. A reduction of 79% in GdE T1-lesions was observed in the lower-dose treatment arm compared with placebo. Additionally, reductions were also observed in new or enlarging T2-lesions and total GdE T1-lesions in both treatment arms.
Based on positive data from the phase II study, Sanofi plans to begin pivotal late-stage studies on frexalimab in multiple sclerosis in early 2024. Sanofi claims frexalimab has a unique mechanism of action as it blocks the CD40/CD40L costimulatory pathway to control MS disease activity without lymphocyte depletion.
J&J Seeks Approval for Single-Tablet Combo for PAH: J&J submitted a new drug application (NDA) to the FDA seeking approval for a single-tablet combination therapy of macitentan 10mg and tadalafil 40mg (M/T STCT) for the long-term treatment of PAH. J&J markets macitentan 10mg under the brand name Opsumit as monotherapy or in combination, indicated for the long-term treatment of PAH. The NDA is based on positive data from J&J’s phase III DUE study, which demonstrated statistically significant improvement in pulmonary hemodynamics (blood flow through pulmonary blood vessels) upon treatment with M/T STCT, compared to macitentan and tadalafil monotherapies in the abovementioned PAH patient population. J&J believes M/T STCT has the potential to improve patient convenience by reducing their pill burden.
EU Approval for Novartis’ Cosentyx for Hidradenitis Suppurativa: The European Commission approved Novartis’ drug Cosentyx for treating hidradenitis suppurativa, a chronic inflammatory skin disease. The European approval was based on data from a phase III study, which showed that treatment with Cosentyx led to rapid symptom relief from as early as Week 4, with response rates continuing to improve up to 1 year. A similar regulatory application seeking approval for Cosentyx for hidradenitis suppurativa indication is under review in the United States, with an FDA decision expected later this year. Cosentyx is presently approved for six inflammatory indications like psoriatic arthritis, plaque psoriasis, ankylosing spondylitis and non-radiographic axial spondyloarthritis and two subtypes of juvenile idiopathic arthritis.
The NYSE ARCA Pharmaceutical Index declined 0.58% in the last five trading sessions.
Large Cap Pharmaceuticals Industry 5YR % Return

Large Cap Pharmaceuticals Industry 5YR % Return
Here’s how the eight major stocks performed in the last five trading sessions.
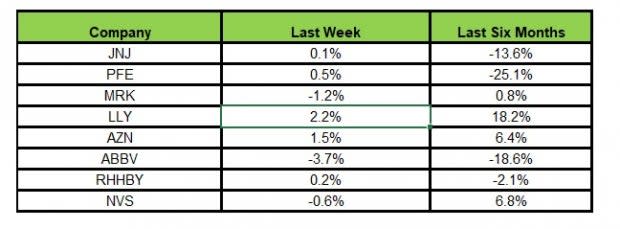
Image Source: Zacks Investment Research
In the last five trading sessions, Lilly rose the most (up 2.2%), while AbbVie declined the most (3.7%).
In the past six months, Lilly has risen the most (18.2%), while Pfizer has declined the most (25.1%).
(See the last pharma stock roundup here: FDA Nod to ABBV’s Rinvoq for New Indication & Other Updates)
What's Next in the Pharma World?
Watch out for regular pipeline and regulatory updates next week.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Sanofi (SNY) : Free Stock Analysis Report
AstraZeneca PLC (AZN) : Free Stock Analysis Report
Johnson & Johnson (JNJ) : Free Stock Analysis Report
Pfizer Inc. (PFE) : Free Stock Analysis Report
Merck & Co., Inc. (MRK) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 