Pharma Stock Roundup: AGN, NVO Report Q1 Earnings, NVS to Buy Takeda's Eye Drug
This week Allergan AGN and Denmark’s Novo Nordisk NVO announced their first-quarter results. Novartis NVS announced the acquisition of the rights to Takeda’s dry eye disease drug, Xiidra for $5.3 billion. Pfizer PFE entered into a deal to buy rare disease private biotech Therachon Holding for an upfront payment of $340 million. Pfizer also gained FDA approval for its rare heart disease candidate tafamidis in two oral formulations, Vyndaqel and Vyndamax.
Recap of the Week’s Most Important Stories
Q1 Earnings Update: Allergan beat estimates for both earnings and sales in the first quarter and marginally raised its guidance for both metrics. Sales were aided by strong performance of key products like Botox and Juvéderm collection of fillers, partially offset by loss of exclusivity on some brands and divestitures of some others in 2018.
Novo Nordisk missed estimates for both earnings and sales in the first quarter. While sales in the Diabetes and Obesity Care segment increased 4% at constant exchange rate (CER), that in the Biopharmaceuticals segment were flat at CER. In 2019, Novo Nordisk expects sales to grow in the range of 2-5% (in local currencies).
Novartis to Buy Xiidra from Takeda in a $5.9B Deal: Novartis announced a deal to buy rights to Japanese drugmaker Takeda’s dry eye disease ophthalmic solution Xiidra 5%, thereby strengthening its ophthalmic pharmaceutical portfolio. Xiidra — a potential blockbuster product — is approved to treat both signs and symptoms of dry eye by inhibiting inflammation caused by the disease. For the rights, Novartis will pay Takeda an upfront payment of $3.4 billion and will be entitled to make potential milestone payments of up to $1.9 billion
Pfizer’s Tafamidis Gets FDA Nod, Buys Rare Disease Biotech: The FDA granted approval to Pfizer’s tafamidis, which has been developed to treat transthyretin amyloid cardiomyopathy (ATTR-CM), a rare and fatal illness associated with progressive heart failure. Pfizer had filed two NDAs for two oral formulations of tafamidis, one for the meglumine form (20 mg capsule) and the other for a free acid form (61 mg capsule). Both the formulations have gained FDA approval to be marketed by the trade names of Vyndaqel and Vyndamax, respectively. The recommended dosage is either Vyndaqel 80 mg orally once-daily, taken as four 20 mg capsules or Vyndamax 61 mg orally once-daily, taken as a single capsule.
Vyndaqel and Vyndamax are the only drugs approved to treat this fatal disease. There are two types of TTR-CM, a hereditary form and a wild-type form of the disease, which is not hereditary. Tafamidis has been approved to treat both the hereditary and the wild-type form of TTR-CM. The FDA’s decision comes much earlier than expected (originally expected in July).
Pfizer also entered into a deal to buy clinical-stage private biotech Therachon Holding for an upfront payment of $340 million. Therachon makes drugs to treat rare diseases and has candidates in its portfolio being evaluated for achondroplasia (TA-46 – phase I) and short bowel syndrome or SBS (apraglutide – phase II). Other than the upfront payment, Pfizer is entitled to pay milestone payments of up to $470 million to Therachon related to development of TA-46. Prior to closing of the transaction, Therachon will spin off its apraglutide development program into an independent company.
Pfizer gained approval for its new cancer medicine, lorlatinib in the EU for previously treated patients with ALK-positive metastatic non-small cell lung cancer (“NSCLC”). In the EU, the drug will be marketed by the trade name of Lorviqua. The drug was approved in the United States by the trade name of Lorbrena in November 2018.
FDA Grants Approval to Expanded Label for Kadcyla: The FDA also granted approval to Roche’s RHHBY breast cancer drug Kadcyla for adjuvant treatment of people with HER2-positive early breast cancer with residual invasive disease after neoadjuvant treatment. Kadcyla, an antibody-drug conjugate, is presently approved as monotherapy in second-line setting for treating metastatic breast cancer in patients who have received treatment with Herceptin or/and taxane.
AstraZeneca’s Calquence Improves Survival in CLL Study: AstraZeneca’s AZN phase III study evaluating Calquence monotherapy compared to standard-of-care medicines for the treatment of relapsed or refractory chronic lymphocytic leukaemia met the primary endpoint. Interim data from the ASCEND study showed that treatment with Calquence led to statistically-significant and clinically-meaningful improvement in progression-free survival (PFS), the primary endpoint. Calquence is presently marketed for the treatment of relapsed or refractory mantle cell lymphoma (MCL). It is being developed for other blood cancers like CLL.
AstraZeneca also gained FDA approval for a new type II diabetes medicine, Qternmet XR which is a combination of its other diabetes drugs Farxiga (dapagliflozin) and Onglyza (saxagliptin) plus metformin.
AstraZeneca and Japan’s Daiichi Sankyo announced positive top-line data from a pivotal phase II study evaluating their antibody drug conjugate (ADC) , trastuzumab deruxtecan in patients with refractory HER2-positive metastatic breast cancer. The study met the primary endpoint. A regulatory application seeking approval for the candidate from the FDA is expected to be filed in the second half of 2019. AstraZeneca acquired joint development and commercialization rights to this promising cancer candidate from Daiichi Sankyo last month.
Merck’s Insomnia Drug Meets Goal in Label Expansion Study: Merck’s MRK Belsomra (suvorexant) C-IV met the primary and secondary efficacy endpoint in phase III study evaluating it for the treatment of insomnia in people with mild-to-moderate Alzheimer’s disease dementia. It was observed that following treatment for four weeks, patients administered Belsomra saw their mean total sleep time (primary endpoint) improve 28.2 minutes versus placebo. Belsomra is presently approved to treat insomnia characterized by difficulties with sleep onset and/or sleep maintenance. Merck plans to file a regulatory application to get approval for inclusion of the above data on the label of the drug.
Sanofi’s Dupixent Gets EU Nod in Asthma: The European Commission granted marketing approval to Sanofi’s Dupixent to treat severe asthma with type 2 inflammation in adults and adolescents 12 years and older. Dupixent was approved for the asthma indication in the United States in October last year.
Bristol-Myers Opdivo Fails in Glioblastoma Study: Bristol-Myers’ late-stage study evaluating Opdivo in combination with radiation in patients with newly diagnosed mgmt-unmethylated glioblastoma multiforme did not meet the primary endpoint of overall survival.
The NYSE ARCA Pharmaceutical Index declined 1.4% in the last five trading sessions.
Large Cap Pharmaceuticals Industry 5YR % Return
Large Cap Pharmaceuticals Industry 5YR % Return
Here is how the seven major stocks performed in the last five trading sessions:
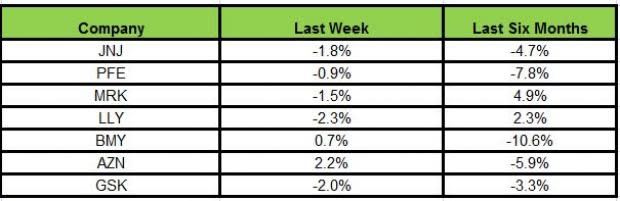
Most of the stocks declined last week except AstraZeneca and Bristol-Myers. While AstraZeneca gained the most (2.2%), Lilly declined the most (2.3%)
In the past six months, Merck has been the biggest gainer (4.9%) while Bristol-Myers declined the most (10.6%).
(See the last pharma stock roundup here: Pharma Stock Roundup: MRK, LLY, PFE Report Q1 Earnings, SNY, ABBV Drugs Get EU Nod)
What's Next in the Pharma World?
Watch out for regular pipeline and regulatory updates next week.
Biggest Tech Breakthrough in a Generation
Be among the early investors in the new type of device that experts say could impact society as much as the discovery of electricity. Current technology will soon be outdated and replaced by these new devices. In the process, it’s expected to create 22 million jobs and generate $12.3 trillion in activity.
A select few stocks could skyrocket the most as rollout accelerates for this new tech. Early investors could see gains similar to buying Microsoft in the 1990s. Zacks’ just-released special report reveals 7 stocks to watch. The report is only available for a limited time.
See 7 breakthrough stocks now>>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Allergan plc (AGN) : Free Stock Analysis Report
Roche Holding AG (RHHBY) : Free Stock Analysis Report
Novartis AG (NVS) : Free Stock Analysis Report
Pfizer Inc. (PFE) : Free Stock Analysis Report
Merck & Co., Inc. (MRK) : Free Stock Analysis Report
AstraZeneca PLC (AZN) : Free Stock Analysis Report
Novo Nordisk A/S (NVO) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 