Pfizer's (PFE) Oral Diabetes Drug Aids in Weight Loss, Stock Up
Pfizer’s PFE shares rose around 5% on Monday after study data showed that its oral type II diabetes candidate, danuglipron, helped patients lose weight faster than Novo Nordisk’s NVO popular diabetes weekly injection, Ozempic.
Data from a phase II study in 411 adults were published in Journal of the American Medical Association (JAMA) Network. The data showed that danuglipron reduced glycated hemoglobin (HbA1c) and fasting plasma glucose (FPG) at all dose levels studied and reduced body weight at doses of 80 mg or more twice daily compared with placebo. The data showed that patients treated with danuglipron lost about 10 pounds over the course of 16 weeks. Novo Nordisk’s Ozempic, a once-daily pre-filled insulin pen, showed a similar weight loss of about 9.9 pounds but over 30 weeks in a similar study.
Novo Nordisk’s Ozempic is currently approved in the United States at 0.5 mg, 1.0 mg and 2.0 mg for the treatment of type II diabetes in adults. Novo Nordisk’s weight loss injection, Wegovy, which was approved by the FDA in June 2021, is the injectable formulation of semaglutide, the same ingredient that is approved as Ozempic for treating type II diabetes.
Danuglipron is an orally administered, small molecule glucagon-like peptide 1 receptor (GLP-1R) agonist. Currently available GLP-1R agonists require subcutaneous administration or strict fasting requirements. In the study, danuglipron’s efficacy and safety profile was consistent with currently available GLP-1R agonists. However, it did not require any subcutaneous administration nor were there any fasting restrictions, the press release mentioned.
This year so far, Pfizer’s stock has declined 24.4% against an increase of 2.6% for the industry.
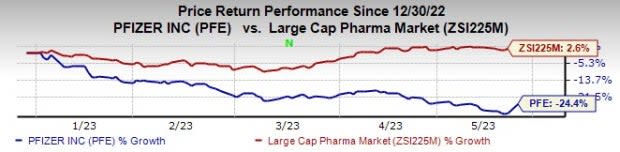
Image Source: Zacks Investment Research
Ozempic is seeing strong demand trends and has witnessed a solid uptake so far. Sales of the drug were up 59% at a constant exchange rate (CER) in the first quarter of 2023. Wegovy sales were up 211% at CER in the first quarter. Novo Nordisk expects sales of Ozempic and Wegovy to pick up in 2023.
On Monday, Novo Nordisk also released data from a phase III study, OASIS 1, which evaluated a once-daily oral formulation of semaglutide in obesity. In the study, oral semaglutide 50 mg achieved 17.4% weight loss at week 68 compared to a reduction of 1.8% with placebo. The weight loss is comparable to that achieved by Wegovy, which is a weekly injection for treating obesity. Novo Nordisk expects to file for regulatory approval of oral semaglutide for obesity in the United States and the EU in 2023
If danuglipron is successful in further studies and eventually approved, it can enjoy strong demand trends as the demand for weight loss drugs is huge and rising. Also, danuglipron is an oral pill versus weekly injections with Ozempic/Wegovy, which is an easier-to-use formulation.
In this context, it is important to mention Eli Lilly’s LLY Mounjaro/tirzepatide, a dual GIP and GLP-1 receptor agonist (GIP/GLP-1 RA), which was launched last year for type II diabetes. Mounjaro is also approved in Europe and Japan. Mounjaro also showed superior weight-loss reduction in clinical studies for the obesity indication. Lilly has initiated a rolling submission in the United States for tirzepatide in obesity that is expected to be completed soon while a regulatory application has already been filed in the EU.
The product is generating impressive sales and already benefiting from strong demand trends for type II diabetes. It is expected to be a key long-term top-line driver for Lilly as it has the potential to be approved for obesity and other diabetes-related diseases.
Zacks Rank
Pfizer currently has a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Pfizer Inc. (PFE) : Free Stock Analysis Report
Novo Nordisk A/S (NVO) : Free Stock Analysis Report
Eli Lilly and Company (LLY) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 