Mirati (MRTX) Seeks EU Nod for Adagrasib to Treat Lung Cancer
Mirati Therapeutics MRTX announced that it has filed a marketing authorization application (MAA) in the European Union (EU) for its KRAS-G12C inhibitor adagrasib for non-small cell lung cancer (NSCLC).
The MAA filed with the European Medicines Agency (EMA) seeks approval for adagrasib as a potential treatment for patients with KRAS-G12C-mutated NSCLC. The patients must have received at least one prior systemic therapy.
MRTX already submitted a new drug application (NDA) with the FDA seeking approval under the accelerated pathway for adagrasib for the above-mentioned indication. In February, Mirati announced that the FDA accepted the NDA and set a PDUFA action date of Dec 14, 2022, for the same.
Shares of Mirati have plunged 59.4% in the year-to-date period compared with the industry’s 24.1% decline.
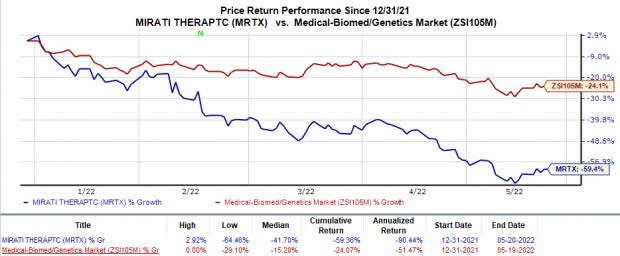
Image Source: Zacks Investment Research
The filings in both the United States and the EU are based on positive top-line data from the phase II potentially registration-enabling monotherapy-cohort of the KRYSTAL-1 study, evaluating adagrasib for the above-mentioned indication. Data from the study was announced last Sepetmber. In the study, treatment with adagrasib led to an objective response rate of 43% and a disease control rate of 80% as of Jun 15, 2021.
Currently, there are very limited options available to patients suffering KRAS-G12C mutated NSCLC. A potential approval of adagrasib will provide Mirati with its first marketed drug and the patients with a new therapeutic option.
Mirati’s adagrasib, if approved, will face stiff competition from Lumakras, a KRAS-G12C inhibitor, marketed by Amgen AMGN. AMGN received accelerated approval for Lumakras as a second-line treatment for locally advanced or metastatic NSCLC from the FDA last May. Earlier in January this year, Amgen also gained approval for the drug in Europe for a similar indication. The drug has shown robust launch uptake since its approval, with Amgen recording revenues worth $62 million from its sales in first-quarter 2022.
The KRYSTAL-1 study is also evaluating adagrasib in multiple cohorts in combination with other therapies. These include a combo therapy of adagrasib with Merck’s MRK Keytruda for first-line NSCLC, a combination of adagrasib plus Boehringer Ingelheim’s Gilotrif (afatinib) for advanced NSCLC and adagrasib combined with Bristol-Myers’ BMY Erbitux for second-line colorectal cancer (CRC).
Preliminary data from the adagrasib plus Merck’s Keytruda cohort demonstrated that the combination achieved a 100% disease control rate, with all seven patients exhibiting tumor regression ranging from 37% to 92% as of Oct 21, 2021.
Mirati is also pursuing a broad combination development program for adagrasib beyond the combinations with Merck’s Keytruda and Bristol-Myers’ Erbitux. These include combinations with SHP2, SOS1 or CDK 4/6 inhibitors.
Both Opdivo and Keutruda are key drivers for Bristol Myers and Merck’s top lines, respectively. During the first quarter of 2022, Bristol Myers recorded $1.9 billion from Opdivo sales while Merck recorded $4.8 billion from Keytruda sales.
Mirati Therapeutics, Inc. Price
Mirati Therapeutics, Inc. price | Mirati Therapeutics, Inc. Quote
Zacks Rank
Mirati currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Bristol Myers Squibb Company (BMY) : Free Stock Analysis Report
Merck & Co., Inc. (MRK) : Free Stock Analysis Report
Amgen Inc. (AMGN) : Free Stock Analysis Report
Mirati Therapeutics, Inc. (MRTX) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 