Intercept (ICPT) Gains on Solid Q2 Results, Ocaliva Recovers
Shares of Intercept Pharmaceuticals, Inc. ICPT gained 9.86% after the company posted impressive performance in second-quarter 2018 following a recovery in sales of lead drug, Ocaliva.
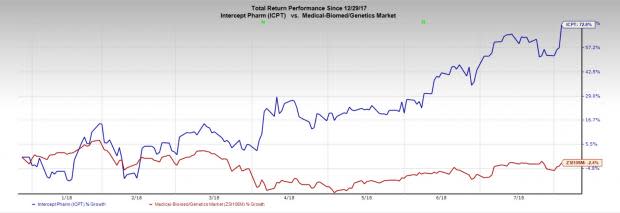
Notably, Intercept’s stock has gained 72.6% in the year so far against the industry’s decline of 2.4%.
Intercept reported a loss of $2.58 per share in the second quarter, narrower than the Zacks Consensus Estimate of a loss of $2.83 and the year-ago loss of $3.46.
Quarterly revenues were $43.6 million, up from $30.8 million in the year-ago quarter and easily surpassing the Zacks Consensus Estimate of $39.9 million.
Quarter in Detail
Ocaliva recorded $43.2 million of sales, up from $35.2 million recorded in the first quarter of 2018 and $30.4 million in the year-ago quarter. Net sales in the United States came in at $34.5 million, while the ex-U.S. Ocaliva net sales came in at $8.7 million.
Ocaliva in combination with ursodeoxycholic (“UDCA”) was approved in the United States, for the treatment of primary biliary cholangitis (“PBC”) in adults with an inadequate response to UDCA, or as monotherapy in adults who are unable to endure UDCA, in 2016. The drug was also granted conditional approval by the European Commission. In February 2018, Ocaliva’s label was updated in the United States, to include a boxed warning and a dosing table that reinforced the existing dosing schedule in PBC patients with Child-Pugh Class B or C or decompensated cirrhosis.
Research and development expenses increased 7.2% year over year to $47.4 million, primarily driven by increases in clinical development programs for Ocaliva. Selling, general and administrative expenses however decreased 2.5% to $65.2 million.
2018 Outlook Reiterated
Ocaliva’s net sales are expected between $170 million and $185 million in 2018. Intercept continues to expect operating expenses in the range of $390-$410 million in 2018.
Pipeline Update
Ocaliva is also being evaluated for other indications including non-alcoholic steatohepatitis (“NASH”) and primary sclerosing cholangitis (“PSC”).
The phase III NASH program includes the REGENERATE trial among patients with advanced fibrosis and the REVERSE trial among patients with compensated cirrhosis. The FDA earlier approved a redesign of the phase III REGENERATE trial on Ocaliva for the safety and efficacy in treating NASH patients with liver fibrosis. The company now needs to achieve only one co-primary endpoint, either fibrosis improvement or NASH resolution compared with the earlier target of achieving both. Enrollment of the interim analysis cohort was completed in the trial and results are expected in the first half of 2019. The REVERSE trial is designed to evaluate the efficacy and safety of Ocaliva in NASH patients with compensated cirrhosis. The trial is currently enrolling.
Our Take
Intercept’s second-quarter results were encouraging as Ocailva sales started to recover. Sales had earlier taken a hit due to the safety issues regarding Ocaliva. Nevertheless, management’s efforts to increase awareness about the updated level and promote Ocaliva, thereafter, is reaping results.
Intercept Pharmaceuticals, Inc. Price, Consensus and EPS Surprise

Intercept Pharmaceuticals, Inc. Price, Consensus and EPS Surprise | Intercept Pharmaceuticals, Inc. Quote
Moreover, the company is looking to expand the drug’s label in the promising NASH space. Per estimates, NASH is expected to surpass hepatitis C as the leading reason for liver transplants in the United States and Europe. NASH market has huge potential and a tentative approval will boost Ocaliva’s prospects, further. The NASH space was in focus in the first half of 2018 with shares of a few companies soaring on positive data on their NASH candidates. Israel-based Galmed Pharmaceuticals GLMD reported positive data on its candidate, Aramchol. The candidate demonstrated statistically significant reduction in liver fat at 52 weeks. This encouraging data boosted investors’ sentiments, leading to the stock’s significant surge. Shares of Madrigal Pharmaceuticals MDGL also soared after the company reported positive 36-week top-line results from a phase II trial on patients with biopsy-proven NASH.
Zacks Rank & Stock to Consider
Intercept currently carries a Zacks Rank #2 (Buy). Another top-ranked stock in the healthcare sector is Gilead Sciences, Inc. GILD, which also sports a Zacks Rank #1 (Strong Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
Gilead’s earnings per share estimates increased from $6.11 to $6.57 for 2018 over the last sixty days. Estimates for 2019 are also up by 12 cents. Shares are up 10% in the year so far.
Will You Make a Fortune on the Shift to Electric Cars?
Here's another stock idea to consider. Much like petroleum 150 years ago, lithium power may soon shake the world, creating millionaires and reshaping geo-politics. Soon electric vehicles (EVs) may be cheaper than gas guzzlers. Some are already reaching 265 miles on a single charge.
With battery prices plummeting and charging stations set to multiply, one company stands out as the #1 stock to buy according to Zacks research.
It's not the one you think.
See This Ticker Free >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Gilead Sciences, Inc. (GILD) : Free Stock Analysis Report
Intercept Pharmaceuticals, Inc. (ICPT) : Free Stock Analysis Report
Galmed Pharmaceuticals Ltd. (GLMD) : Free Stock Analysis Report
Madrigal Pharmaceuticals, Inc. (MDGL) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 
