AstraZeneca shares slide amid vaccine doubts and watchdog assessment
AstraZeneca (AZN.L) shares continued to slide on Friday as its CEO Pascal Soriot announced that the UK-based pharmaceutical giant is likely to launch a new global trial to test its coronavirus vaccine at a lower dosage.
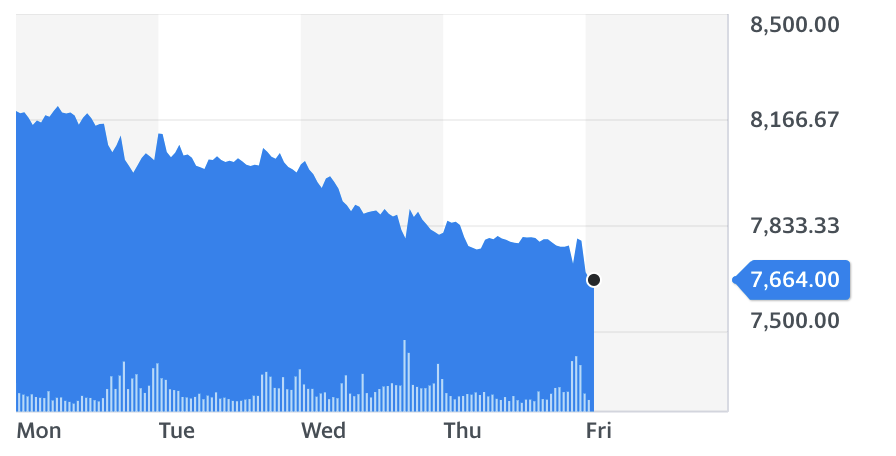
For the fifth consecutive trading session, AstraZeneca shares fell — this time by over 1% in the opening trading hour, as the UK government also confirmed on Friday that a medical watchdog is now weighing up whether to sign off the jab with Oxford University for a rollout.
The potential new trial comes after the surprise discovery that the vaccine was more effective in trial participants who received a dose and a half-dose, when they were supposed to received two.
Trials in Brazil and the UK showed a 90% effectiveness rate, compared to a 62% rate for those who received two doses. But AstraZeneca did not initially acknowledge the different dosages were an error, sparking greater scrutiny of the results.
WATCH: UK regulator to assess vaccine for rollout
Pascal Soriot told Bloomberg: “Now that we’ve found what looks like a better efficacy we have to validate this, so we need to do an additional study.”
He said it was likely to be another international trial, but the CEO said it would be “faster” than the previous one. “We know the efficacy is high so we need a smaller number of patients.”
But he said he did not expect it to delay securing regulators’ approval in the UK and the EU.
READ MORE: AstraZeneca and Oxford vaccine hopes lift European stocks
The UK government has now formally asked the Medicines and Heathcare products Regulatory Agency (MHRA) to assess the Oxford and AstraZeneca jab’s suitability for emergency rollout. It requested a similar evaluation of the vaccine produced by Germany’s BioNTech (BNTX) and US pharmaceutical giant Pfizer (PFE) a week ago.
“We are working tirelessly to be in the best possible position to deploy a vaccine as soon as one is approved by the independent regulator the MHRA,” said health and social care secretary Matt Hancock.
The UK’s department of health has written to the MHRA asking it to assess whether the vaccine’s rollout could be fast-tracked. A clause in UK regulations allows the temporary authorisation of unlicensed medicines in response to public health emergencies.
Moncef Slaoui, scientific head of the US government’s vaccine rollout programme Operation Warp Speed, said this week AstraZeneca's more effective lower-dose vaccine had only been given to trial participants under 55.
"The dosing error has arguably been both a blessing and a headache for AZD1222," wrote analysts at Shore Capita, noting it had sparked both the discovery and confusion.
"People need to know what’s the actual efficacy of the product they’re receiving, which means confirming the half dose/full dose arm, if that’s the one that is to be taken forward to market.
"Critically, because people may opt to take a different vaccine based on different efficacy and safety profiles, and they may behave differently if they feel the protection on offer is less with one vaccine versus another."
WATCH: Why can't governments just print more money?

 Yahoo Finance
Yahoo Finance 