Biogen (BIIB), Eisai's Alzheimer Drug Likely to Get Full FDA Nod
The FDA issued briefing documents on Biogen’s BIIB Alzheimer’s disease (“AD”) drug Leqembi (lecanemab) for the upcoming Peripheral and Central Nervous System Drugs Advisory Committee (“PCNSDAC”) Meeting on Jun 9. The drug has been developed in collaboration with Eisai, who is leading the clinical development.
The FDA briefing document acknowledges that treatment with Leqembi in the early stages of the disease reduced the rate of clinical decline in AD patients. This is supported by data from the CLARITY AD study, which achieved its primary endpoint of reduction in Clinical Dementia Rating-Sum of Boxes (“CDR-SB”) scale by 27% compared with a placebo. The CDR-SB is a numerical scale that measures the severity of symptoms of dementia. The study also achieved statistically significant results for its secondary endpoints.
The PCNSDAC is set to give a recommendation on whether the data from the phase III CLARITY AD study confirms the clinical benefit of Leqembi for treating AD. The committee will also consider the benefit/risk assessment of the drug in treating AD.
An anti-amyloid beta protofibril antibody, Leqembi was approved by the FDA under the accelerated pathway earlier this January. The accelerated approval was based on data from a phase II study (Study 201), which showed that treatment with lecanemab reduced the accumulation of amyloid beta (Aβ) plaque in the brain. Aβ plaque is a lesion in the brain that defines the neuropathological diagnosis of AD.
The day that Biogen/Eisai received accelerated approval for Leqembi, Eisai filed a supplemental biologics license application (“sBLA”) seeking full/traditional approval for Leqembi in AD on the same day. This sBLA, currently under FDA review, is supported by data from the CLARITY-AD study. A final decision from the regulator is expected on Jul 6, 2023.
If Leqembi receives a traditional approval from the FDA, it also becomes eligible for a broader coverage under the United States government’s Medicare plan. Currently, Medicare restricts coverage of drugs like Leqembi, approved under the accelerated pathway only to those enrolled in CMS-approved clinical studies.
Shares of Biogen were up 1.7% on Jun 7, as based on the above observation, investors expect a positive recommendation from the FDA Committee on Leqembi approval. In fact, the stock has increased 10.1% against the industry’s 7.3% decline.
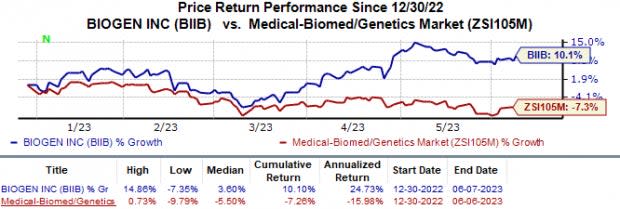
Image Source: Zacks Investment Research
Biogen developed lecanemab in collaboration with Eisai, with the latter leading the clinical development and regulatory submissions. Eisai and Biogen will co-commercialize and co-promote the drug. The companies also developed another anti-amyloid antibody, Aduhelm, which was approved by the FDA in June 2021 but failed to generate significant sales.
Some other pharma companies like Eli Lilly LLY, Prothena PRTA and Roche RHHBY are also developing their antibody candidates targeting the AD indication.
Eli Lilly developed donanemab, an investigational antibody therapy, for AD. Last month, Lilly announced positive data from the phase III TRAILBLAZER-ALZ 2 study that showed that treatment with donanemab significantly slowed cognitive and functional decline in people with early symptomatic AD. Based on this result, Lilly will start filing regulatory submissions for the candidate in AD indication, including a regulatory filing with the FDA by June 2023-end.
Prothena is evaluating multiple AD candidates in early-stage studies targeting AD indication. Prothena is evaluating PRX005, an investigational antibody that targets tau, a protein implicated in diseases — including AD, frontotemporal dementia, progressive supranuclear palsy, chronic traumatic encephalopathy and other tauopathies. Data from an early-stage study evaluating PRX005 is expected by the year-end.
Prothena is also evaluating another investigational high-potency monoclonal antibody PRX012, designed to target a key epitope at the N-terminus of Aβ for treating AD, in a phase I study. PRTA plans to initiate an IND filing for PRX123, a dual Aβ-Tau vaccine treatment and prevention therapy for AD, by the end of this year.
Roche’s phase III GRADUATE I and II studies on a key Alzheimer’s pipeline candidate, gantenerumab failed to meet the primary endpoint of slowing clinical decline in people with early AD in November 2022. The level of beta-amyloid removal by gantenerumab was less than expected. Roche developed the candidate in collaboration with MorphoSys.
Zacks Rank
Biogen currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Biogen Inc. (BIIB) : Free Stock Analysis Report
Roche Holding AG (RHHBY) : Free Stock Analysis Report
Eli Lilly and Company (LLY) : Free Stock Analysis Report
Prothena Corporation plc (PRTA) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 