BD's (BDX) New FDA Approval to Improve Vaginitis Testing
Becton, Dickinson and Company BDX, popularly known as BD, recently received the FDA’s 510(k) clearance for the BD Vaginal Panel on the BD COR System. The panel is a comprehensive diagnostic test that directly detects the three most common infectious causes of vaginitis using BD's high-throughput molecular diagnostic platform for large laboratories.
The BD Vaginal Panel is the third assay available for use on the BD COR System in the United States, the first being the BD Onclarity HPV (human papillomavirus) assay and the second being the BD CTGCTV2 molecular assay.
The latest regulatory approval is expected to solidify BD’s foothold in the global Molecular Diagnostics business, thereby boosting its overall Life Sciences segment.
A Few Words About the Panel
The BD Vaginal Panel was originally granted marketing authorization for the BD MAX System in 2016. The panel is the first microbiome (the community of microorganisms, such as fungi, bacteria and viruses, that exists in a particular environment)-based polymerase chain reaction assay that uses a single swab and test to simultaneously detect organisms associated with bacterial vaginosis (BV), vulvovaginal candidiasis (VVC) and Trichomonas vaginalis (TV). The test reports a clear positive or negative result for each condition separately.
The 510(k) clearance for the BD Vaginal Panel on the BD COR System is the first high-throughput version of the test.
Significance of the Approval
The accurate diagnosis of BV, VVC and TV is critical for ensuring appropriate treatment routines and decreasing the risk of associated complications and resistance to treatment. The use of a single test can also aid in reducing the need for repeat testing and unnecessary use of treatments as well as lower the risk of contracting sexually transmitted infections (STIs).
The three most prevalent non-viral STIs are Chlamydia trachomatis (CT), Neisseria gonorrhoeae (GC) and Trichomonas vaginalis (TV), which can also have overlapping symptoms. With the clearance of BD Vaginal Panel on the BD COR System, labs are now expected to offer physicians both BD Vaginal Panel and BD CTGCTV2 testing from one swab and one patient collection.
Further, if a test is positive for VVC (commonly known as a yeast infection), the BD Vaginal Panel is currently the only FDA-cleared Nucleic Acid Amplification Test that provides separate results for the two Candida fungi species (C. glabrata and C. krusei) that are resistant to traditional antimicrobials, thereby ensuring proper treatments.
Per management, a recent study has shown that many women did not receive the appropriate diagnosis and treatment for their vaginitis symptoms after an initial physician visit. This made them schedule a new appointment due to persistent symptoms. Management believes that the BD Vaginal Panel will likely end the cycle of repeated visits, misdiagnosis and ineffective treatment for millions of women suffering from vaginitis.
Industry Prospects
Per a report by MarketsandMarkets, the global molecular diagnostics market is anticipated to reach $30.2 billion by 2027 from $23.2 billion in 2022 at a CAGR of 5.4%. Factors like the emergence of new viruses, technological advancements in molecular diagnostics and the growing awareness of early disease diagnosis are likely to drive the market.
Given the market potential, the latest regulatory clearance is expected to significantly strengthen BD’s business worldwide.
Recent Developments in Life Sciences Arm
Last month, BD received the FDA’s approval for the BD Onclarity HPV Assay to be used with the ThinPrep Pap Test. The two most common Pap vials currently used by laboratories in the United States are the BD SurePath Liquid-based Pap Test vial and the Hologic ThinPrep Pap Test PreservCyt Solution vial.
The same month, BD reported its first-quarter fiscal 2023 results, wherein it registered an improvement in the overall base revenues. BD Life Sciences segment saw the Integrated Diagnostic Solutions unit’s strong growth in the base business (driven by strong demand for BD’s respiratory testing portfolio, which was partly aided by the timing of dealer orders, strong BD Kiestra lab automation installations and leveraging its higher BD MAX installed base for molecular In Vitro Diagnostic assay growth) and strength in the Biosciences unit.
Price Performance
Shares of BD have lost 9.2% in the past year compared with the industry’s 9.6% decline and the S&P 500's 12.5% fall.
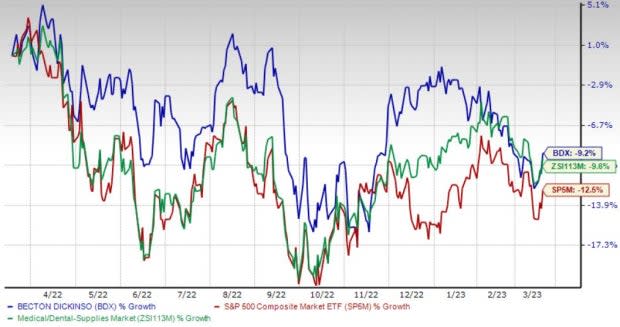
Image Source: Zacks Investment Research
Zacks Rank & Other Key Picks
Currently, BD carries a Zacks Rank #2 (Buy).
A few other top-ranked stocks in the broader medical space are Hologic, Inc. HOLX, Henry Schein, Inc. HSIC and Avanos Medical, Inc. AVNS.
Hologic, carrying a Zacks Rank #2 at present, has an estimated long-term growth rate of 15.2%. HOLX’s earnings surpassed the Zacks Consensus Estimate in all the trailing four quarters, the average beat being 30.6%.
You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Hologic has gained 4.6% against the industry’s 17.2% decline in the past year.
Henry Schein, carrying a Zacks Rank #2 at present, has an estimated long-term growth rate of 8.1%. HSIC’s earnings surpassed estimates in three of the trailing four quarters and matched the same in the other, the average beat being 2.9%.
Henry Schein has lost 10.4% compared with the industry’s 9.6% decline over the past year.
Avanos, carrying a Zacks Rank #2 at present, has an estimated growth rate of 1.8% for 2023. AVNS’ earnings surpassed estimates in all the trailing four quarters, the average beat being 11%.
Avanos has lost 13.5% compared with the industry’s 17.2% decline over the past year.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Becton, Dickinson and Company (BDX) : Free Stock Analysis Report
Hologic, Inc. (HOLX) : Free Stock Analysis Report
Henry Schein, Inc. (HSIC) : Free Stock Analysis Report
AVANOS MEDICAL, INC. (AVNS) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 