Apellis (APLS) Rises on FDA Acceptance of NDA Amendment for GA
Apellis Pharmaceuticals, Inc. APLS announced that the FDA has accepted its unsolicited major amendment to the new drug application (“NDA”) for its targeted C3 therapy, pegcetacoplan, for the treatment of geographic atrophy (“GA”) secondary to age-related macular degeneration (“AMD”), a leading cause of blindness.
A decision from the regulatory body is now expected on Feb 26, 2023. The FDA also reaffirmed that it is not planning to hold an advisory committee meeting to discuss the above application.
Earlier this month, APLS announced plans to submit the 24-month efficacy data from the phase III DERBY and the phase III OAKS studies as part of its NDA application. The data showed increasing and consistent effects with every-other-month and monthly treatments with pegcetacoplan as well as a favorable safety profile in both studies.
Shares of Apellis were up 16.7% on Friday following the announcement of the news. The stock has improved 6.7% so far this year against the industry’s decline of 19.2%.
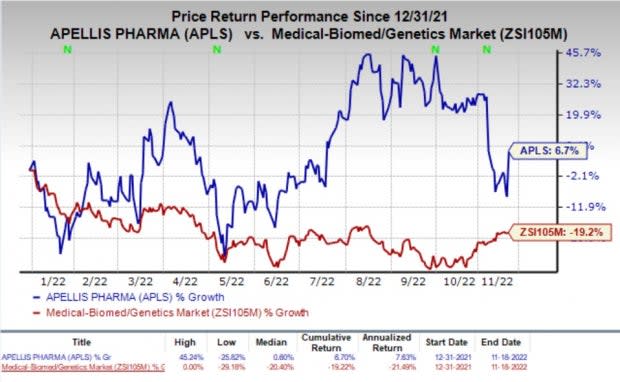
Image Source: Zacks Investment Research
In July, the FDA accepted and granted priority review to the NDA for pegcetacoplan to treat GA secondary to AMD. A decision from the regulatory body was expected on Nov 26, 2022. However, the latest major amendment to the NDA for pegcetacoplan extended the review period by three months.
A marketing authorization application for pegcetacoplan to treat GA is expected to be filed by 2022-end with the European Medicines Agency.
In May 2021, the FDA approved pegcetacoplan (marketed as Empaveli) as a monotherapy treatment for adult patients suffering from PNH.
Empaveli is approved for treatment-naïve patients and those switching from Alexion’s [now part of AstraZeneca AZN] C5 inhibitor therapies for PNH, namely Soliris and Ultomiris (ravulizumab).
AZN closed the acquisition of rare-disease drugmaker Alexion for $39 billion in July 2021, strengthening its immunology franchise.
The European Commission approved pegcetacoplan (marketed as Aspaveli) to treat adult patients with PNH who are anemic after treatment with a C5 inhibitor for at least three months in December 2021.
In the first nine months of 2022, Empaveli/Aspaveli generated sales worth $45.4 million.
Several label expansion studies on pegcetacoplan are currently underway. Potential approval for GA and additional indications will boost sales and drive growth for the company in the days ahead.
Zacks Rank & Stocks to Consider
Apellis currently carries a Zacks Rank #3 (Hold). Some better-ranked stocks in the biotech sector are Angion Biomedica Corp. ANGN and Syndax Pharmaceuticals, Inc. SNDX, both sporting a Zacks Rank #1 (Strong Buy) at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
Loss per share estimates for Angion Biomedica have narrowed 6.1% for 2022 and 3.9% for 2023 in the past 60 days.
Earnings of Angion Biomedica surpassed estimates in three of the trailing four quarters and missed the mark on the other occasion. ANGN witnessed an earnings surprise of 66.42% on average.
Loss per share estimates for Syndax Pharmaceuticals have narrowed 4.9% for 2022 and 10.1% for 2023 in the past 60 days.
Earnings of Syndax Pharmaceuticals surpassed estimates in three of the trailing four quarters and met the same on the other occasion. SNDX witnessed an earnings surprise of 95.39% on average.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
AstraZeneca PLC (AZN) : Free Stock Analysis Report
Angion Biomedica Corp. (ANGN) : Free Stock Analysis Report
Syndax Pharmaceuticals, Inc. (SNDX) : Free Stock Analysis Report
Apellis Pharmaceuticals, Inc. (APLS) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 