AbbVie (ABBV), J&J to Withdraw 2 Blood Cancer Nods for Imbruvica
AbbVie ABBV and Johnson & Johnson JNJ announced they would voluntarily withdraw the accelerated approvals granted by the FDA to Imbruvica (ibrutinib) in mantle cell lymphoma (MCL) and marginal zone lymphoma (MZL) indications. This decision was made in consultation with the FDA.
This decision to voluntarily withdraw two approvals was based on data from two separate phase III confirmatory studies, which evaluated Imbruvica in MCL and MZL indications, respectively. Per the FDA, data from both confirmatory studies were insufficient to support conversion to full approval.
In the year so far, shares of AbbVie have registered breakeven growth while J&J’s stock price has declined 6.5%. During the same period, the industry has risen 0.7%.
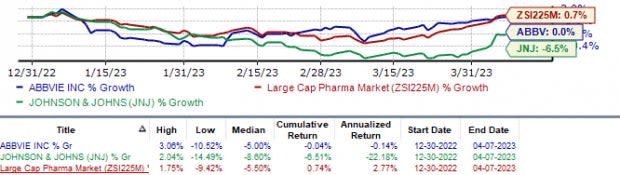
Image Source: Zacks Investment Research
The phase III SHINE study and phase III SELENE study served as confirmatory studies which evaluated Imbruvica in MCL and MZL indications, respectively. Although the SHINE study did achieve its primary endpoint of progression-free survival (PFS), study participants who were administered the drug in addition to chemoimmunotherapy reported increased adverse reactions compared to the placebo-controlled arm. The SELENE study did not achieve its primary endpoint of PFS.
The FDA had granted accelerated approval to Imbruvica for treating MCL patients who have received at least one prior therapy in 2013. The agency granted another accelerated approval to Imbruvica in 2017 to treat MZL patients who require systemic therapy and received at least one prior anti-CD20-based therapy. These accelerated approvals were granted based on overall response rates in separate phase II studies.
The above decision will not affect FDA approval for Imbruvica in other indications. Apart from MCL and MZL, Imbruvica is currently approved for chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL), Waldenström’s macroglobulinemia (WM) and chronic graft-versus-host disease (cGVHD) indications.
Imbruvica has been developed by AbbVie in collaboration with J&J. Per the terms of collaboration, the companies jointly market Imbruvica in the United States while J&J has exclusive rights for marketing the drug outside the country. J&J shares international profits earned from Imbruvica with AbbVie.
In the last few quarters, sales of Imbruvica have been declining steadily amid rising competition from novel oral treatments in the United States. The drug is facing stiff competition from AstraZeneca’s AZN Calquence and BeiGene’s Brukinsa, which the FDA has approved to treat similar indications for which Imbruvica is already approved.
Currently, AstraZeneca’s Calquence is approved by the FDA as capsule and tablet formulations to treat CLL/SLL and MCL indications. For fiscal 2022, AstraZeneca generated $2.1 billion from Calquence product sales. BeiGene’s Brukinsa is currently approved by the FDA in CLL/SLL, WM, MCL and MZL indications.
AbbVie Inc. Price
AbbVie Inc. price | AbbVie Inc. Quote
Johnson & Johnson Price
Johnson & Johnson price | Johnson & Johnson Quote
Zacks Rank & Stocks to Consider
Both AbbVie and J&J currently carry a Zacks Rank #3 (Hold). A better-ranked stock in the overall healthcare sector includes Novo Nordisk NVO, which sports a Zacks Rank #1 (Strong Buy) at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
In the past 60 days, estimates for Novo Nordisk’s 2023 earnings per share have increased from $4.20 to $4.51. During the same period, the earnings estimates per share for 2024 have risen from $4.90 to $5.26. Shares of Novo Nordisk are up 17.8% in the year-to-date period.
Earnings of Novo Nordisk beat estimates in three of the last four quarters while missing the mark on one occasion. On average, the company’s earnings witnessed a surprise of 3.00%. In the last reported quarter, Novo Nordisk’searnings beat estimates by 2.47%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
AstraZeneca PLC (AZN) : Free Stock Analysis Report
Johnson & Johnson (JNJ) : Free Stock Analysis Report
Novo Nordisk A/S (NVO) : Free Stock Analysis Report
AbbVie Inc. (ABBV) : Free Stock Analysis Report